Alternaria alternata
Basics
Alternaria is a genus comprising approximately 50 species {3318; 816; 813}. Among these, A. alternata is one of the most common and most studied fungi of the aeromycota {813; 989}. Alternaria is recognised worldwide as a common plant pathogen and airborne allergen: it is the typical aeroallergen species of the genus and in a majority of cases, is the most frequent species associated with human and animal health problems.
Taxonomy
| Kingdom | Fungi | Order | Pleosporales |
| Phylum | Ascomycota | Family | Pleosporaceae |
| Class | Euascomycetes (or Dothideomycetes) | Genus | Alternaria |
Comoclathris permunda is the sexual form of Alternaria alternata and Lewia sp. is the sexual form of many other Alternaria spp. {3842}; other teleomorph genera are Graphyllium and Pleospora.
It is said that there are 44 definite, well-known species, but there may be hundreds more; in fact the Universal Protein Resource data library lists 95 named species in its registry among 433 registered strains {3318}. Some authorities also suggest that A. alternata is a species complex rather than a single species and could consist of several heterogeneous species {816}.
Habitat/Ecology
Alternaria spp. are cosmopolitan dematiaceous fungi commonly isolated from plants, soil, food and indoor air environment. Alternaria species are known as major plant pathogens {1635; 725} and saprophytes on many substrates {816; 1056; 3729; 725}. They are ubiquitous in the environment and are a natural part of the fungal flora worldwide. They are normal agents of vegetable matter decomposition. The spores are airborne and found in the soil and water, as well as indoors. They are often found in a large proportion of air samples but at concentrations below 10% of total counts.
More details
A. alternata is a worldwide saprophyte, commonly found on plants, foodstuffs and textiles, soil and indoor air environment {1056}; decaying wood, wood pulp and compost are also favourite substrates for this species {725}. A. alternata is a seed plant pathogen {1635}, found most commonly on weakened plants {813}; it is able to damage cereals and vegetables in the field but rarely during storage. Alternaria can often be the source of black spots on tomatoes, onions and carrots {725} and can cause early potato blight as well as infest other plants.
This mould is considered one of the most important outdoor airborne moulds, along with Cladosporium {2644; 2649}; it reaches peak concentrations during late summer {2388} and is usually always in greater concentrations in outdoor air than in indoor air {2216}. For example, among fungal species found on leaf surfaces of trees in Tulsa (Oklahoma),Alternaria represented 4.69% of total fungi isolated; during peak leaf concentrations, air sample concentrations reached 7.5 CFU/m3 , although far below Phoma and Cladosporium spp.{1281}.
Interestingly, there are many reports linking thunderstorm activity to an increase in airborne levels of Alternaria species; indeed, an association was reported between A. alternata sensitivity in patients with asthma following thunderstorms {Nasser, 2009 3878 /id} {1570}.
In the wake of hurricanes Katrina and Rita in 2005 on the Gulf Coast of Louisiana, thousands of homes were flooded and a characterisation of airborne moulds revealed a high prevalence of Alternaria spp.in both outdoor samples (45%) and in indoor air (20%) {695}.
In Crete, following a 10-year aerobiological study, 18 mould species exhibited a yearly seasonal pattern, including Alternaria spp., with a prevalence of 6.2% {162}.
In a New South Wales city in Australia, Alternaria was one of the most frequent taxa identified when measuring personal exposure to outdoor spores {618}. In the same study, the number of spores inhaled by volunteers was also measured, with Alternaria beingamong the most frequent.
In the outdoor air of Athens, viable spores of Alternaria species accounted for 6.32% of total fungal spores, being the third most abundant species after Cladosporium spp. and Penicillium spp., with an annual mean concentration of 34 CFU/m3 {1282}.
Outdoor air sampling of several areas of Lithuanian urban areas revealed that A. alternata was the third most common airborne fungal species identified, with an occurrence of 62.99% {2226}.
In the Netherlands, aerobiological samples for fungal spores taken from a hospital rooftop revealed the presence of 21 fungal species: while Alternaria ranked 7th, its concentration represented only 0.9% of the overall total, with an average of 2.2 CFU/m3 {864}.
In Saudi Arabia, downfall dust collected from the roofs of numerous houses and taxis revealed that Alternaria was recovered in moderate occurrence, with a total count on agar plates ranging between 5 700 and 6 800 CFU/g dry air-dust particles {930}. In the same country, Kwaasi et al. reported Alternaria as one of the most frequent isolates found in sampled aeroallergens in sandstorm dust {1841}.
In Egypt, other studies conducted in similar conditions also measured low concentrations of Alternaria despite its high prevalency: Alternaria was recovered in 40 to 70% of air-dust particle samples collected on rooftops of selected houses, being among the most frequently detected moulds. However, concentrations only represented 3.9 to 8.4 CFU/mg air-dust of the total spore count {929}.
In Yokohama, Japan, Alternaria spp. accounted for 22.7 to 48.6 % of total airborne fungi in outdoor air samples, whereas a lower prevalence was recovered in indoor air (6.0 to 17.5%) of two apartments of two four-story buildings {2517}.
Growth requirements
Alternaria grows on a variety of substrates. Temperature requirements are between 2 and 32 °C (optimum 25-29 °C) {1056; 725}. The optimal pH is between 4 -5.4, although this species may grow on a pH range of 2.7 to 8 {989}. The minimum available water (Aw) requirements range between 0.85 and 0.88 depending on the species {813; 989}.
Water Activity : Aw = 0.85 – 0.88
Growth on building materials or indoor environment
Alternaria sp. has been reported worldwide in the indoor environment in air, surface and dust samples {1584; 624; 2388; 605; 1756; 2193}. Alternaria is a secondary colonizer {587} and grows on various building materials and indoor substrates. It is often found on floors, in carpets and mattress dust; it can establish itself on damp window frames and window putty, walls and ceilings, wallpaper and acrylic paint finishes as well as in humidifier water and ventilation filters; it also contaminates textiles (cotton, jute, wool), rubber, magnetic tapes, negatives and oil paintings, paper, parchment and certain synthetic materials {989; 1056; 3729; 725}.
Alternaria is a frequent contaminant in water-damaged or flooded buildings {695; 720}.
More details
In a controlled study on mould growth on wet gypsum wallboard in an indoor environment, Alternaria appeared as a secondary colonizer, being detected more than a month after immersing the building materials in water {587}. In the wake of hurricanes Katrina and Rita in 2005 on the Gulf Coast of Louisiana, thousands of homes were flooded and a characterisation of airborne moulds revealed a high prevalence of Alternaria spp. in both outdoor samples (45%) and in indoor air (20%) {695}.
In a study of 831 housing units in 75 different locations throughout the U.S., Alternaria antigens were detected in the vast majority (95-99%) of indoor dust samples {1085}. In Texas, samples from houses of 100 patients mainly suffering from respiratory symptoms were analysed for the presence of moulds; cultures revealed the presence of Alternaria in all homes, as well as Aspergillus, Penicillium and Cladosporium {705}. A 13-month study of work-leisure-sleep environments of 130 allergy patients from New York state detected 20 species of airborne mould {1821}, with Alternaria ranking 7th with a prevalence of 44% of all samples grown.
On the other hand, a Danish study of 72 mould-contaminated building material samples, collected from 23 public buildings, arrived to a different conclusion: in this study, Alternaria was found to be one of the least frequently encountered fungi (6%) {605}.
In 22 Spanish homes of patients diagnosed with respiratory allergy to moulds, air sampling (431 indoor and 150 outdoor exposed plates) revealed that A. alternata was the most frequent species isolated, detected in 37.1% of indoor air samples (as compared to 62.6% in outdoor air); in fact, this species was the only fungus for which a difference between indoor and outdoor air was observed {624}.
Sampling of 49 houses in Santa Fe (Argentina) revealed a moderate prevalence of Alternaria of 8.68% and a mean count of 39.81 CFU/m3, i.e. 24.6 CFU/m3 in urban area, 63.1 CFU/m3 in suburban areas {1584}.
In a Korean study measuring outdoor and indoor air levels (42 bars, 41 internet cafes and 44 schools) for bacteria and fungi {2388}, Alternaria species was the fourth most common fungus and was present in nearly 100% of sampling locations (indoor or outdoor). Concentrations ranged from non detectable to 100 CFU/m3. In many locations, indoor concentrations were higher than outdoor concentrations, leading the authors to postulate a possible difference in certain characteristics of these microenvironments.
Similar findings have also been obtained in warm climates. For example, in Saudi Arabia, Alternaria species were also found in household indoor environments of Riyadh (between 33 CFU/g and 49 CFU/g house dust) {1756}. This fungus was identified in water closet air of a university in Egypt, and detected in 40% to 56% of samples (20) at concentrations ranging from 56 to 90 CFU/agar plate (exposed 5 minutes) {2193}.
Alternaria sp. was also detected in one automobile air conditioning system, among 29 sampled cars in Atlanta {2288}.
Air samples, collected from a seven-storey building undergoing renovations revealed that the prevalence of Alternaria increased from 4% prior to renovating to 13% after renovations; moreover, this mould was also one of the most abundant fungal genera, accounting for 11.2% of total airborne spores in this study (27 CFU/m3) {1790}.
Laboratory section
Normal laboratory precautions should be exercised in handling cultures of this species within Biosafety Level 2 practices and containment facilities.
Colony, macroscopic morphology
Alternaria grows rapidly and matures within 5 days {412}; colony size reaches a diameter of 3 to 9 cm following incubation at 25 °C for 7 days on potato glucose agar (PGA) {816}. The colony is flat, downy to woolly and covered by greyish, short, aerial hyphae, becoming in time greenish black or olive brown with a light border {412}. The reverse side is typically brown to black due to pigment production {816; 412}. When grown on malt extract agar (MEA) at 25 °C, colonies reach a diameter of 6 cm within 7 days, being black or greyish {989; 725}. On potato carrot agar (PCA) medium at 25 °C they attain a diameter of 5.5 to 6.0 cm in 7 days; the mycelium is hyaline with black or olivaceous-black conidia {1056}.
Microscopic morphology
Hyphae are septate and dark. Conidiophores are short (40-70 x 3-4 µm), also septate and brown in color, occasionally producing a zigzag appearance. The simple or branched conidophores bear large ovoid or ellipsoid conidia (7-10 x 23-34 µm), which have both transverse and longitudinal septations. These conidia may produce germ tubes and are ovoid to obclavate, darkly pigmented, muriform, smooth or roughened. The end of the conidium nearest the conidiophore is round while it tapers towards the apex, hence giving the conidia a typical beak or club-like appearance {816; 989; 412}.
Specific metabolites
Organics compounds (including VOCs)
There are no reports of volatile organic compounds specific to Alternaria sp. that could be detrimental to human or animal health.
In the indoor environment of damp buildings, many metabolites, including microbial volatile organic compounds (mVOCs), have been identified as common to several fungal species. The frequent presence of Alternaria in mouldy buildings could contribute to the concentration of these metabolites indoors {3717} and to the occurrence of sick building syndrome {569; 2649}.
Some very potent enzymes produced by Alternaria species, especially cellulases, easily decompose the cellulose components of the contaminated material {725}. Some species also produce proteases and amylases {989}. The production of a melanin-like pigment is one of the characteristic metabolites of Alternaria sp.
Mycotoxins
Alternaria spp. produce a unique group of mycotoxins, including alternariols, altenuenes, altertoxins and tenuazonic acid {813; 989; 603; 725}. These mycotoxins may be produced in tomatoes, apples, olives, wheat, sorghum, sunflower seeds and pecans. Tenuazonic acid is an important mycotoxin that is also produced by another fungus, Phoma sorghina {715}.
Building materials artificially contaminated with a variety of fungi, including Alternaria sp,, have been studied for their toxic properties {603}; five out of six isolates of Alternaria spp. produced alternariol and alternariol monomethyl ether.
The production of mycotoxins by Alternaria alternata on cellulosic ceiling tiles was examined by thin-layer chromatography and high-performance liquid chromatography. Alternariol and alternariol monomethyl ether were found in ceiling tile extracts, whereas extracts of control rice cultures of all three isolates also produced these mycotoxins in addition to altenuene and altertoxin-I. Extensive fungal growth and mycotoxin production occurred in these ceiling tiles at a relative humidity of 84-89% and 97% {796}. In controlled studies, 25°C appeared as the optimum temperature for the production of the 3 toxins alternariol, alternariol methyl ether and alternaric acid {3873}; hence suggesting that indoor conditions can be favourable to toxin production.
Adverse health reactions
Irritation and inflammation
In the indoor environment of damp buildings, many organic compounds, including microbial volatile organic compounds (mVOCs), have been identified associated with several fungal species. Some of these compounds are common to most fungal species and probably contribute to various health problems associated with indoor air quality. However many of the fungal metabolites identified are non reactive and are in low concentrations in indoor air {594}.
Alternaria produces some mVOCs that are common to many moulds {3717; 3858; 1840; 2749} and may contribute to the occurrence of irritation symptoms observed in sick building syndrome {569; 2649}. The complete specific mVOC profile of Alternaria alternata is not yet published.
Allergic reactions
Alternaria spp. components are recognised potent allergens. The relatively large abundance of Alternaria conidia in outdoor air and its occurrence in mouldy houses makes this fungus one of the most important fungal allergen sources {989; 39; 2355; 2285; 808}. This worldwide allergen generates high prevalence sensitisation: depending on the geography and populations studied, the prevalences of positive skin tests (ST) are 3% to 12% in the general population and 13% to 39.4% in atopic subjects (Simon-Nobbe 2007 {3911}). In one study conducted in a large cohort of patients presenting respiratory symptoms (asthma and allergic rhinitis), a 66% incidence of Alternaria sensitisation was observed in the group of patients that had one or more positive fungal ST {3207}.
In particular, Alternaria alternata sensitisation is responsible for Type I allergic reactions {1635; 2285}, rhinitis {648}, asthma and allergic sinusitis {2558; 3802; 166}. A. alternata is associated with asthma exacerbations {2641; 824; 1780; 3896; 3910; 3904; 3907} and even with onset of asthma {3902}; life threatening exacerbations have sometimes been associated with Alternaria sp. {1085}.
More details
Alternaria sp. has also been reported to be an aetiological cause of chronic rhinosinusitis (CRS) {2678; 1469; 2558}. In one study, Alternaria was reported to be isolated more frequently in rhinosinusitis patients, but could also be present in healthy individuals {2678}. Furthermore, in the case of CRS, a non-IgE-mediated immunological mechanism of reactivity to Alternaria and other common fungi has been described {1469; 191}.
Many studies have shown a correlation between Alternaria sensitisation and severity of asthma attacks: in fact, it appears that an association can be made with the concentration of airborne Alternaria which in turn can be associated with climate zones and meteorological phenomena {3878; 1570; 1780}. A study of the short term association between daily fungal spore concentrations and indicators of daily asthma exacerbations in a large urban population was conducted in London, UK {1780}. Among children 14 years old and younger, it was found that the number of individual spores, including Alternaria spores, was associated with increased attendance to hospital emergency departments; no such association was observed in adults.
Many studies have been conducted worldwide in order to determine the prevalence of Alternaria sensitisation resulting from both outdoor and indoor exposure. In the general population, between 3 and 4% of patients tested in the U.S. and Scandinavia have a positive reaction to Alternaria allergens {1635}. These prevalences are often 5 to 10 times greater when studying allergic patients.
In the United States, 34.5% of asthmatic patients have a positive skin test to Alternaria specific antigens as revealed by a study performed on 12 106 patients {2640}. A study conducted in Kansas, USA, reported an association between elevated mean concentrations of certain fungi, including Alternaria, and respiratory health symptoms (reported on a health basis questionnaire) {1814}. As part of the National Survey of Lead and Allergens in Housing throughout the United States, an indoor air quality study was conducted in order to determine the association between indoor Alternaria contamination and asthma: a study of samples from 831 housing units inhabited by 2456 individuals revealed that the prevalence of current symptomatic asthma increased with increasing Alternaria concentrations {648}.
In European countries, prevalence of sensitisation to Alternaria is typically between 3 and 30% {2544}. In studying 105 asthma patients treated in Poland, Niedoszytko et al. {1585} found that 24% of asthma patients had a positive skin prick test reaction to Alternaria, as compared to 13% in the control group; however, in this study, exacerbation of asthma symptoms was mostly associated with other moulds. In a 10-year aerobiological study in Crete, the highest prevalence of positive skin prick tests among 571 atopic patients was 11.9% for Alternaria spp. {162}. In another Greek study examining rates of sensitisation to fungal spores of 5 different species, including Alternaria, skin tests (skin prick tests) revealed that of the 1311 patients tested, 13.5% had a positive reaction to this fungus, showing the highest rate among the 5 species tested {1788}.
Allergic components and mechanism
The many biochemical studies and pathophysiologial modelling of Alternaria allergenic properties reflect the importance of this ubiquitous fungus. The major allergens of A. alternata have been isolated and 13 recognised fractions have been well characterised; moreover, it has been shown that wherever A. alternata conidia have been isolated in the world, they always contain the same allergens. Alt a 1 is the major allergen: it is responsible for 98% of A. alternata sensitisations {2642}. Closely related moulds, such as Cladosporium herbarum and Ulocladium species, also contain some allergenic fractions identical to those found in A. alternata {3760; 3912; 725}. At least 35 Alternaria species, other than A. alternata, have been characterised as allergens and have been shown to cross react within the phylum {3912}.
Immunological mechanisms involved in IgE production have been extensively investigated {3911} {3877; 2676; 2642; 3905; 3799}.
More details
It has been proposed that some pathologies may have IgE and IgG-mediated mechanisms. In a study of chronic rhinosinusitis (CRS) patients in which immune mechanisms were studied in vitro at the cellular level, fungal-specific immunity characterised by serum IgG, but not IgE, distinguished the subgroups of CRS patients with eosinophilic mucus (EMCRS) from control groups regardless of the presence of fungus in the EM or of systemic fungal allergy. Fungal-specific IgE responses in fungal-allergic EMCRS were no different to those observed in fungal-allergic controls, thus challenging the presumption of a unique pathogenic role of fungal allergy in "allergic fungal sinusitis" {191}.
Some pathologies may be the result of combined exposure to Alternaria and to bacteria.
For example, in patients with rhinosinusitis associated with Alternaria, fungal extracts alone resulted in minimal changes in cytokine expression levels in peripheral blood lymphocytes. However, staphylococcal superantigen B (BSE) increased the expression of IFN-gamma, an effect which was magnified by the combined addition of SEB and fungal extracts to the culture medium {2650}.
Toxic effects (mycotoxicosis)
Ingestion of Alternaria toxins has been implicated in animal and in human health disorders {3748} but very few cases are reported in the literature. Surveys to date have shown that occurrence in food is very low, hence there are currently no official guideline limits on these mycotoxins.
Outbreaks of mycotoxicosis in farm animals in Germany have been linked to cereals contaminated by Alternaria toxins.
Moreover, contamination of cereal grains by Alternaria mycotoxins could explain the aetiology of Kashin-Beck Disease (KBD). A non-identified Alternaria metabolite has been detected in barley samples stemming from symptomatic families {Haubruge, 2001 3847 /id}; however, the role of Alternaria could be only secondary to that of Trichothecium roseum and Dreschlera sp. which may be more closely associated with this disease {1838, Chasseur, C., 2001}.
Occupational acute toxic lung injury has been attributed to Alternaria alternata in early stages of organic dust toxic syndrome (ODTS) {2672}.
Alternaria mycotoxicosis through inhalation has yet to be confirmed. Lower respiratory symptoms in children exposed to a mouldy indoor environment have been well documented. Whether fungal toxin exposure contributes to this association is uncertain. Some authors suggest that these lower respiratory illnesses in children and some sick building syndrome cases could be in part attributable to fungal components of Alternaria (glucans and proteins) and to mycotoxin exposures, although actual mechanisms remain unknown {1087}.
However, many controlled studies have been undertaken either in vitro at the cellular level or in animal models and have characterised the toxic properties of these Alternaria metabolites.
More details
Alternaria toxins are cytotoxic in vitro to mammalian and bacterial cells and are feototoxic and teratogenic in mice and hamsters; these toxins are known to block synthesis of sphingolipids {1635}. Two isolates of Alternaria spp. were found to be toxic, damaging livers of mice under laboratory conditions; however, in the same study, other strains exhibited no toxicity {1771}. In one study testing the ciliostatic activity of extractable endometabolites and exometabolites of 5 strains of filamentous fungi isolated from mouldy walls of a dwelling, results showed that species of Alternaria were able to produce compounds with a very high ciliostatic activity on chick trachea {1840}.
Alternariols and altenuenes are weakly toxic to mice but are cytotoxic to cultured human cell lines in vitro at concentrations between 6 and 28 µg/ml. Altertoxins and the related compound stemphyltoxin-III, also produced by Alternaria, are mutagenic {Brugger, 2006 3929 /id}. The toxic mechanism of tenuazonic acid appears to be one by which protein synthesis is inhibited by preventing newly formed protein molecules from detaching from the ribosomes on which they were formed {3748, Lawley, R., 2009; 3483, Pohland, A.E., 1993}.
Infections and colonisations
While infection with Alternaria is seldom reported, it can no longer be considered as rare. Alternaria spp. have emerged as opportunistic pathogens particularly in immunosuppressed patients, such as transplant recipients and patients with haematologic malignancies and other cancers {2671; 2031; 1792; 3808}{Morrison, 1993 3931 /id}. In these immunocompromised patients, simple colonisation may lead to the development of invasive disease {816}.
Alternaria alternata may cause upper respiratory infections in AIDS patients {3809}.
Alternaria sp. is considered one of the causative agents of phaeohyphomycosis {2865}. Cases of onychomycosis, sinusitis, ulcerated cutaneous and subcutaneous infections, and keratitis, as well as deep sited soft tissue infections and osteomyelitis due to Alternaria sp. have been reported {3832; 1598; 2645; 2683; 2674; 2684; 3818; 3821; 3817; 3671; 3811; 3807; 3800; 166}{Schell, 2000 3932 /id}.
More details
In immunocompetent patients, Alternaria colonises the paranasal sinuses, leading to chronic hypertrophic sinusitis. In immunocompromised patients, the colonisation may lead to the development of invasive disease {3805}{816}.
Fungal colonisation and infection of the eye by Alternaria species have been reported after surgical interventions, although the incidence is very low {2643; 2684}. Alternaria infection progressing to the brain has been described in extremely rare cases {725}.
Onychomycosis accounts for up to 50% of all nail infections and is often caused by dermatophytes fungi. However, other fungi are also involved; in a mycological study of onychomycosis in 88 patients, Veer et al. {2683} reported that Alternaria spp. were responsible for 16.6% of cases. Hence, cutaneous alternariosis can no longer be considered a rare fungal infection {2685} and some cases of subcutaneous phaeohyphomycosis caused by Alternaria have been reported {2681; 2674}; a case of oral mucosal phaeohyphomycosis has also been described {2647}.
Virulence factors
No special virulence factor has been reported. Important subcutaneous lesions and nail infections are associated with Alternaria, even though most species do not grow at 37 °C {813}.
Specific settings
Nosocomial infections
Alternaria has been found in the hospital setting {313; 386}, but no confirmed case of nosocomial infection due to Alternaria has been reported in the literature. However some authors consider that opportunistic infections due to Alternaria could become an emerging problem for immunocompromised patients in the health care setting {1747; 366}.
More details
In a large tertiary hospital with extensive indoor renovation and extensive demolition, 74 duct dust and air samples were collected for Aspergillus surveillance purposes: notably, the viable fungal samples also revealed the presence of Alternaria spp, with a mean indoor air concentration of 0.79 CFU/m3 (as compared to 8.04 in outdoor air) {313}.
In another study involving seven hospitals in the Eastern United States, eleven air filters from heating, ventilation, and air-conditioning systems, selected on the basis of discoloration, were analysed: filters from 3 of the 7 hospitals were found to be contaminated with A. alternata {386}.
Occupational diseases
In the workplace, Alternaria sp. has been associated with occupational asthma in bakers {2001}{2401; 2989} and with organic dust toxic syndrome (ODTS) reactions in cotton handlers {2672}. In the agricultural setting as well as in compost and sewage treatment facilities {855}, high concentrations of Alternaria on plant or waste material may increase the risk for Type I allergic reactions and Type III HP or farmer’s lung disease {725}{1777; 1778; 2680; 2658; 1822}. Alternaria inhalation may also be associated with woodworker’s lung and apple store hypersensitivity xxxxxxxxxxxxxxxx {813}.
In some instances, workers handling plant material could be at higher risk for superficial infections due to introduction of this fungus through skin abrasions or puncture wounds {2546}.
More details
Baker’s asthma is considered to be linked with inhalation of Alternaria conidia present in flour; Orman et al. reported that 4.1% and 3.2% of workers in industrial or home type bakeries respectively showed positive skin tests to Alternaria antigens {2001}. This species was also recovered in air samples of a rural bakery in India, showing a moderate concentration between 7.3 CFU/m3and 86.7 CFU/m3, except during the month of July in the packing section, where its concentration reached 2033 CFU/m3 {2401}. In industrial and home type bakeries, Orman et al. reported an occurrence of Alternaria spp. between 1.1% and 5.8% {2001}.
A case of severe organic dust toxic syndrome was reported in a worker exposed to natural cotton powder for 3 weeks {2672} and associated with A. alternata contaminated cotton. However, in a Bristish study, contents of cotton samples from several regions of the world were examined for their role in organic dust syndrome: Alternaria was only a minor contaminant in one sample from one country and was not identified as a likely source of occupational health problems for cotton workers {700}.
In rural areas, fungal species constitute a major component of environmental contaminants in facilities where animals are housed {1778; 2331}. In Slovakian poultry feed mixtures, A. alternata was found with a prevalence of 20% of samples tested {1778}In Lithuania, Alternaria spp. were isolated from a poultry house and represented 2.1 to 5.5% of all species isolated {854}. In a large rural indoor cattle shed in India, the frequency detection of A. alternata in airborne samples was low (0.48 – 2.0%), with concentrations of up to 52 CFU/m3 {2373}. In an other Indian study, Alternaria was commonly found inside grain storage facilities {1777}.
The numerous studies underlining the presence of Alternaria in rural settings suggest that this mould could potentially increase the risk of respiratory problems in farm workers; however, in actual fact, very few articles confirm this risk. Two cases of farmer’s lung disease (IgG mediated) due to Alternaria have been reported {725}. In agricultural field workers, Alternaria has been identified as one of the causative agents of otitis media directly related to occupation {Wadhwani, 1984 3933 /id}.
An important airborne concentration of this fungus has been also associated with processing of peppermint and chamomile herbs {2680}, and with flax scotching farms {2658}.
Alternaria alternata can also reside in municipal sewage treatment plants as shown in a Polish study in which the mould was found in 10.4% of samples {855}.
In greenhouses, A. alternata may be found on sick or dead plants {725}. A study of the indoor air quality of greenhouses and dwellings with house plants revealed that Alternaria was the single most identified species with the highest prevalence, with an occurrence between 52% and 82% of homes with house plants and between 80% and 100% of samples from a botanical garden {1822}.
Flournoy et al. also reported that Alternaria spp. were the most prevalent species isolated from rose thorns (45 isolates/103 plants) {2546}; rose plants are notoriously susceptible to fungal diseases and may act as important vehicles in the transmission of pathogenic microorganisms via punctures penetrating the skin.
Dental unit handpieces are a source of microbial aerosol. A study conducted at 25 dental unit sites revealed a concentration of 24.8 CFU/m3 of A. alternata species prior to disinfection and 16.4 CFU/m3after standard disinfection procedures. A. alternata accounted for nearly 13% of total fugal concentration, thus ranking second among the species identified {2396}.
In a study designed to determine the association between respiratory symptoms and allergic sensitisation (measured by skin tests) in 214 office workers, results established that respiratory symptoms were significantly associated with exposure to Alternaria allergens detected in heating, ventilation and air conditioning systems {2666}.
Renovation and remodelling activities conducted in buildings may also release high concentrations of particulate matter, viable and non-viable spores into the air. Air samples collected from a seven-storey building undergoing renovation revealed that the frequency of Alternaria increased from 4% prior to renovating to 13% after renovation; this mould was also one of the most abundant fungal genera, accounting for 11.2% of total airborne spores (27 CFU/m3) {1790}.
Diagnostic tools
Cultures
Since Alternaria species are cosmopolitan and ubiquitous in nature, they are also common laboratory contaminants. Thus, their isolation in culture requires cautious evaluation {1819}. The presence of Alternaria in sinus fluids may be compatible with chronic rhinosinusitis, but can also be a fortuitous finding {2678}.
Histopathology
Dark coloured filamentous hyphae are observed in Alternaria-infected tissue sections stained with H&E. If pigment formation is not obvious, Fontana-Masson silver stain, which is specific to melanin, may be applied {816}.
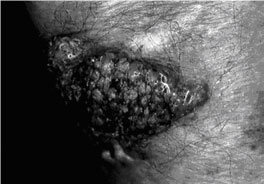
Vegetating lesion on forearm, with mammiliate surface, resulting from a subcutaneous lesion caused by Alternaria alternata {2674}.
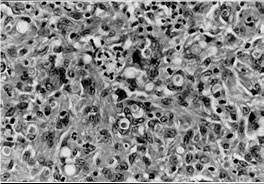
Fungal structures in a granulomatous inflammation in the dermis caused by A. alternata Periodic acid-Schiff after diastase digestion (x 1700) {2674}.
Immunodiagnosis
The Alternaria extracts for in vitro and in vivo testing are available commercially and can be useful in establishing an association between an allergic reaction (IgE) or hypersensitivity pneumonitis (IgG) and Alternaria exposure. Alternaria allergen extracts are part of the basic testing panels as single antigens or as part of antigen pools. The most common commercially available allergen preparations for use in vivo and/or in vitro are: Alternaria sp. extracts for in vivo testing, i.e. skin tests, and Alternaria alternata alt-1 for in vitro testing such as RAST.
| Test | IgE | IgG | Antigens | Other |
|---|---|---|---|---|
| Skin Tests | X | |||
| RAST-IgE | X | |||
| RAST-IgG | X | |||
| ELISA-ELIFA | ||||
| Immunodiffusion | ||||
| Immunofluorescence | Experimental | |||
| Complement fixation | ||||
| PCR | Experimental | |||
| Other |
More details
Alternaria allergen extracts are part of a FDA «Biological Product Deviation Reporting Non-Blood Product Codes» surveillance program. FDA-listed antigens are as follows {3285}:
GJ06 - Alternaria solani
GJ07 - Alternaria tenuis (syn. alternata)
Bibliography
- 39. Horner, W. E. (2003). Assessment of the indoor environment: evaluation of mold growth indoors. Immunol.Allergy Clin North Am.2003.Aug.;23(3):519.-31. 23, 519-531.
- 162. Guneser, S., Atici, A., Koksal, F., and Yaman, A. (1994). Mold allergy in Adana, Turkey. Allergol.Immunopathol.(Madr.). 22[2], 52-54.
- 166. Manning, S. C., Schaefer, S. D., Close, L. G., and Vuitch, F. (1991). Culture-positive allergic fungal sinusitis. Arch Otolaryngol.Head Neck Surg. 117[2], 174-178.
- 191. Pant, H., Kette, F. E., Smith, W. B., Wormald, P. J., and Macardle, P. J. (2005). Fungal-specific humoral response in eosinophilic mucus chronic rhinosinusitis. Laryngoscope. 115[4], 601-606.
- 277. Baur, X., Richter, G., Pethran, A., Czuppon, A. B., and Schwaiblmair, M. (1992). Increased prevalence of IgG-induced sensitization and hypersensitivity pneumonitis (humidifier lung) in nonsmokers exposed to aerosols of a contaminated air conditioner. Respiration. 59[4], 211-214.
- 313. Curtis, L., Cali, S., Conroy, L., Baker, K., Ou, C. H., Hershow, R., Norlock-Cruz, F., and Scheff, P. (2005). Aspergillus surveillance project at a large tertiary-care hospital. J Hosp.Infect. 59[3], 188-196.
- 366. Groll, A. H. and Walsh, T. J. (2001). Uncommon opportunistic fungi: new nosocomial threats. Clin Microbiol Infect. 7 Suppl 2:8-24., 8-24.
- 386. Simmons, R. B., Price, D. L., Noble, J. A., Crow, S. A., and Ahearn, D. G. (1997). Fungal colonization of air filters from hospitals. Am Ind.Hyg.Assoc.J. 58[12], 900-904.
- 412. Larone, D H. (1987). Medically important fungi. A guide to identification. 2nd edition, -230 p. New York - Amsterdam - London, Elsevier Science Publishing Co., Inc.
- 569. McGrath, J. J., Wong, W. C., Cooley, J. D., and Straus, D. C. (1999). Continually measured fungal profiles in sick building syndrome. Curr.Microbiol. 38[1], 33-36.
- 581. Pharmacia Diagnostics AB. (2007). Allergy & autoimmunity. Diagnostics product catalogue 2007. internet , 1-48. Pharmacia.
- 587. Krause, M., Geer, W., Swenson, L., Fallah, P., and Robbins, C. (2006). Controlled study of mold growth and cleaning procedure on treated and untreated wet gypsum wallboard in an indoor environment. J Occup.Environ Hyg. 3[8], 435-441.
- 594. Claeson, A. S., Levin, J. O., Blomquist, G., and Sunesson, A. L. (2002). Volatile metabolites from microorganisms grown on humid building materials and synthetic media. J Environ Monit. 4[5], 667-672.
- 603. Nielsen, K. F., Gravesen, S., Nielsen, P. A., Andersen, B., Thrane, U., and Frisvad, J. C. (1999). Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia. 145[1], 43-56.
- 605. Gravesen, S., Nielsen, P. A., Iversen, R., and Nielsen, K. F. (1999). Microfungal contamination of damp buildings--examples of risk constructions and risk materials. Environ Health Perspect. 107 Suppl 3:505-8., 505-508.
- 618. Green, B. J., O'meara, T., Sercombe, J., and Tovey, E. (2006). Measurement of personal exposure to outdoor aeromycota in northern New South Wales, Australia. Ann Agric.Environ Med. 13[2], 225-234.
- 624. de Ana, S. G., Torres-Rodriguez, J. M., Ramirez, E. A., Garcia, S. M., and Belmonte-Soler, J. (2006). Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. J Investig.Allergol.Clin Immunol. 16[6], 357-363.
- 648. Salo, P. M., Arbes, S. J., Jr., Sever, M., Jaramillo, R., Cohn, R. D., London, S. J., and Zeldin, D. C. (2006). Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 118[4], 892-898.
- 695. Rao, C. Y., Riggs, M. A., Chew, G. L., Muilenberg, M. L., Thorne, P. S., Sickle, D. V., Dunn, K. H., and Brown, C. (2007). Characterizing airborne molds, endotoxins and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl.Environ Microbiol..
- 700. Lane, S. R. and Sewell, R. D. (2006). The fungal profile of cotton lint from diverse sources and implications for occupational health. J Occup Environ Hyg. 3[9], 508-512.
- 705. Rea, W. J., Didriksen, N., Simon, T. R., Pan, Y., Fenyves, E. J., and Griffiths, B. (2003). Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch Environ Health. 58[7], 399-405.
- 715. Fiedler, K., Schutz, E., and Geh, S. (2001). Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int J Hyg.Environ Health. 204[2-3], 111-121.
- 720. Taskinen, T., Meklin, T., Nousiainen, M., Husman, T., Nevalainen, A., and Korppi, M. (1997). Moisture and mould problems in schools and respiratory manifestations in schoolchildren: clinical and skin test findings. Acta Paediatr. 86[11], 1181-1187.
- 725. Gravesen, S., Frisvad, J. C., and Samson, RA. (1994). Microfungi. 1st edition, -168 p. Copenhagen, Munksgaard.
- 808. Al-Doory, Y and Domson, JF. (1984). Mould allergy. Lea & Febiger.
- 813. EMLAB. (2007). Environmental Microbiology Laboratory, Inc. (EMLab): An index of some commonly encountered fungal genera.
- 816. Patterson, T. F., McGinnis, M. R., and ed. (2009). The fungi :description. Site Doctor Fungus. Mycoses Study Group.
- 824. Denning, D. W., O'Driscoll, B. R., Hogaboam, C. M., Bowyer, P., and Niven, R. M. (2006). The link between fungi and severe asthma: a summary of the evidence. Eur Respir.J. 27[3], 615-626.
- 854. Lugauskas, A., Krikstaponis, A., and Sveistyte, L. (2004). Airborne fungi in industrial environments--potential agents of respiratory diseases. Ann Agric.Environ Med. 11[1], 19-25.
- 855. Prazmo, Z., Krysinska-Traczyk, E., Skorska, C., Sitkowska, J., Cholewa, G., and Dutkiewicz, J. (2003). Exposure to bioaerosols in a municipal sewage treatment plant. Ann Agric.Environ Med. 10[2], 241-248.
- 864. Beaumont, F., Kauffman, H. F., de Monchy, J. G., Sluiter, H. J., and de Vries, K. (1985). Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. II. Comparison of aerobiological data and skin tests with mould extracts in an asthmatic population. Allergy. 40[3], 181-186.
- 929. Abdel-Hafez, S. I., Shoreit, A. A., Abdel-Hafez, A. I., and el Maghraby, O. M. (1986). Mycoflora and mycotoxin-producing fungi of air-dust particles from Egypt. Mycopathologia. 93[1], 25-32.
- 930. Abdel-Hafez, S. I. and Shoreit, A. A. (1985). Mycotoxins producing fungi and mycoflora of air-dust from Taif, Saudi Arabia. Mycopathologia. 92[2], 65-71.
- 989. Centre de recherche sur la conservation des documents graphiques. (2007). Moisissures et biens culturels. Ministère de la culture et de la Communication, France.
- 995. Integrated Laboratory Systems. (2004). Stachybotrys chartarum (or S. atra or S. alternans) [CAS No. 67892-26-6]; Review of Toxicological Literature. National Institute of Environmental Health Sciences (NIEHS). 1-66. United States.
- 1056. Samson, RA, Hoekstra, ES, and Frisvad, JC. (2004). Introduction to food and airbone fungi. 7th, -389 p. Baarn, Centralalbureau voor Schimmellcultures, Institute of the Royal Netherlands Academy of Arts and Sciences.
- 1085. Salo, P. M., Yin, M., Arbes, S. J., Jr., Cohn, R. D., Sever, M., Muilenberg, M., Burge, H. A., London, S. J., and Zeldin, D. C. (2005). Dustborne Alternaria alternata antigens in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 116[3], 623-629.
- 1087. Stark, P. C., Burge, H. A., Ryan, L. M., Milton, D. K., and Gold, D. R. (2003). Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am J Respir.Crit Care Med. 168[2], 232-237.
- 1281. Levetin, E. and Dorsey, K. (2006). Contribution of leaf surface fungi to the air spora. Aerobiologia 22[1], 3-12.
- 1282. Pyrri, I. and Kapsanaki-Gotsi, E. (2007). A comparative study on the airbone fungi in Athens, Greece, by viable and non-viable sampling methods. Aerobiologia 23, 3-15.
- 1469. Ponikau, J. U., Sherris, D. A., Kephart, G. M., Adolphson, C., and Kita, H. (2006). The role of ubiquitous airborne fungi in chronic rhinosinusitis. Clin Rev Allergy Immunol. 30[3], 187-194.
- 1481. Schwarze, P. E., Ovrevik, J., Lag, M., Refsnes, M., Nafstad, P., Hetland, R. B., and Dybing, E. (2006). Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 25[10], 559-579.
- 1570. Pulimood, T. B., Corden, J. M., Bryden, C., Sharples, L., and Nasser, S. M. (2007). Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 120[3], 610-617.
- 1584. Basilico, Mde L., Chiericatti, C., Aringoli, E. E., Althaus, R. L., and Basilico, J. C. (2007). Influence of environmental factors on airborne fungi in houses of Santa Fe City, Argentina. Sci Total Environ. 376[1-3], 143-150.
- 1585. Niedoszytko, M., Chelminska, M., Jassem, E., and Czestochowska, E. (2007). Association between sensitization to Aureobasidium pullulans (Pullularia sp) and severity of asthma. Ann Allergy Asthma Immunol. 98[2], 153-156.
- 1598. Xie, L., Zhai, H., Shi, W., Zhao, J., Sun, S., and Zang, X. (2007). Hyphal Growth Patterns and Recurrence of Fungal Keratitis after Lamellar Keratoplasty. Ophthalmology.
- 1635. De Lucca, A. J. (2007). Harmful fungi in both agriculture and medicine. Rev Iberoam.Micol. 24[1], 3-13.
- 1675. Greenberger, P. A. (2004). Mold-induced hypersensitivity pneumonitis. Allergy Asthma Proc. 25[4], 219-223.
- 1747. Tomsikova, A. (2002). Causative agents of nosocomial mycoses. Folia Microbiol (Praha). 47[2], 105-112.
- 1756. Bokhary, H. A. and Parvez, S. (1995). Fungi inhabiting household environments in Riyadh, Saudi Arabia. Mycopathologia. 130[2], 79-87.
- 1771. Gupta, J., Pathak, B., Sethi, N., and Vora, V. C. (1981). Histopathology of Mycotoxicosis produced in Swiss albino mice by metabolites of some fungal isolates. Appl Environ Microbiol. 41[3], 752-757.
- 1777. Chattopadhyay, B. P., Das, S., Adhikari, A., and Alam, J. (2007). Exposure to varying concentration of fungal spores in grain storage godowns and its effect on the respiratory function status among the workers. Ind Health. 45[3], 449-461.
- 1778. Labuda, R. and Tancinova, D. (2006). Fungi recovered from Slovakian poultry feed mixtures and their toxinogenity. Ann Agric Environ Med. 13[2], 193-200.
- 1780. Atkinson, R. W., Strachan, D. P., Anderson, H. R., Hajat, S., and Emberlin, J. (2006). Temporal associations between daily counts of fungal spores and asthma exacerbations. Occup Environ Med. 63[9], 580-590.
- 1788. Gioulekas, D., Damialis, A., Papakosta, D., Spieksma, F., Giouleka, P., and Patakas, D. (2004). Allergenic fungi spore records (15 years) and sensitization in patients with respiratory allergy in Thessaloniki-Greece. J Investig.Allergol.Clin Immunol. 14[3], 225-231.
- 1790. Abdel Hameed, A. A., Yasser, I. H., and Khoder, I. M. (2004). Indoor air quality during renovation actions: a case study. J Environ Monit. 6[9], 740-744.
- 1792. Safdar, A., Singhal, S., and Mehta, J. (2004). Clinical significance of non-Candida fungal blood isolation in patients undergoing high-risk allogeneic hematopoietic stem cell transplantation (1993-2001). Cancer. 100[11], 2456-2461.
- 1814. Su, H. J., Rotnitzky, A., Burge, H. A., and Spengler, J. D. (1992). Examination of fungi in domestic interiors by using factor analysis: correlations and associations with home factors. Appl Environ Microbiol. 58[1], 181-186.
- 1819. Pritchard, R. C. and Muir, D. B. (1987). Black fungi: a survey of dematiaceous hyphomycetes from clinical specimens identified over a five year period in a reference laboratory. Pathology. 19[3], 281-284.
- 1821. Rogers, S. A. (1984). A 13-month work-leisure-sleep environment fungal survey. Ann Allergy. 52[5], 338-341.
- 1822. Burge, H. A., Solomon, W. R., and Muilenberg, M. L. (1982). Evaluation of indoor plantings as allergen exposure sources. J Allergy Clin Immunol. 70[2], 101-108.
- 1838. Chasseur, C., Suetens, C., Michel, V., Mathieu, F., Begaux, F., Nolard, N., and Haubruge, E. (2001). A 4-year study of the mycological aspects of Kashin-Beck disease in Tibet. Int Orthop. 25[3], 154-158.
- 1840. Pieckova, E. and Jesenska, Z. (1998). Molds on house walls and the effect of their chloroform-extractable metabolites on the respiratory cilia movement of one-day-old chicks in vitro. Folia Microbiol (Praha). 43[6], 672-678.
- 1841. Kwaasi, A. A., Parhar, R. S., al-Mohanna, F. A., Harfi, H. A., Collison, K. S., and al-Sedairy, S. T. (1998). Aeroallergens and viable microbes in sandstorm dust. Potential triggers of allergic and nonallergic respiratory ailments. Allergy. 53[3], 255-265.
- 2001. Orman, A., Ficici, S. E., Ay, A., Ellidokuz, H., Sivaci, R. G., and Konuk, M. (2005). Detection of fungi spectrum in industrial and home bakeries and determinated fungal allergy with skin prick test. Asian Pac J Allergy Immunol. 23[2-3], 79-85.
- 2031. Sorensen, J., Becker, M., Porto, L., Lambrecht, E., Schuster, T., Beske, F., Rickerts, V., Klingebiel, T., and Lehrnbecher, T. (2006). Rhinocerebral zygomycosis in a young girl undergoing allogeneic stem cell transplantation for severe aplastic anaemia. Mycoses. 49 Suppl 1:31-6., 31-36.
- 2193. Ismail, M. A. and Abdel-Sater, M. A. (1994). Mycoflora inhabiting water closet environments. Mycoses. 37[1-2], 53-57.
- 2216. Temprano, J., Becker, B. A., Hutcheson, P. S., Knutsen, A. P., Dixit, A., and Slavin, R. G. (2007). Hypersensitivity pneumonitis secondary to residential exposure to Aureobasidium pullulans in 2 siblings. Ann Allergy Asthma Immunol. 99[6], 562-566.
- 2226. Lugauskas, A., Sveistyte, L., and Ulevicius, V. (2003). Concentration and species diversity of airborne fungi near busy streets in Lithuanian urban areas. Ann Agric Environ Med. 10[2], 233-239.
- 2285. Horner, W. E., Helbling, A., Salvaggio, J. E., and Lehrer, S. B. (1995). Fungal allergens. Clin Microbiol Rev 8[2], 161-179.
- 2288. Simmons, R. B., Noble, J. A., Rose, L., Price, D. L., Crow, S. A., and Ahearn, D. G. (1997). Fungal colonization of automobile air conditionning systems. Journal of Industrial Microbiology & Biotechnology 19, 150-153.
- 2331. van, Halderen A., Green, J. R., Marasas, W. F., Thiel, P. G., and Stockenstrom, S. (1989). A field outbreak of chronic aflatoxicosis in dairy calves in the western Cape Province. J S Afr.Vet.Assoc. 60[4], 210-211.
- 2355. Levetin, E. and Horner, W. E. (2002). Fungal aerobiology: exposure and measurement. Chem Immunol. 81:10-27., 10-27.
- 2373. Adhikari, A., Sen, M. M., Gupta-Bhattacharya, S., and Chanda, S. (2004). Volumetric assessment of airborne fungi in two sections of a rural indoor dairy cattle shed. Environ Int. 29[8], 1071-1078.
- 2388. Jo, W. K. and Seo, Y. J. (2005). Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools, and homes. Chemosphere. 61[11], 1570-1579.
- 2396. Szymanska, J. (2006). Exposure to airborne fungi during conservative dental treatment. Ann Agric Environ Med. 13[1], 177-179.
- 2401. Adhikari, A., Sen, M. M., Gupta-Bhattacharya, S., and Chanda, S. (2000). Incidence of allergenically significant fungal aerosol in a rural bakery of West Bengal, India. Mycopathologia. 149[1], 35-45.
- 2517. Takahashi, T. (1997). Airborne fungal colony-forming units in outdoor and indoor environments in Yokohama, Japan. Mycopathologia. 139[1], 23-33.
- 2544. Farruggia, E. and Bellia, M. (2001). [Occupational allergic asthma in greenhouses. Report of a clinical case]. Med Lav. 92[3], 203-205.
- 2546. Flournoy, D. J., Mullins, J. B., and McNeal, R. J. (2000). Isolation of fungi from rose bush thorns. J Okla.State Med Assoc. 93[7], 271-274.
- 2558. Karpovich-Tate, N., Dewey, F. M., Smith, E. J., Lund, V. J., Gurr, P. A., and Gurr, S. J. (2000). Detection of fungi in sinus fluid of patients with allergic fungal rhinosinusitis. Acta Otolaryngol. 120[2], 296-302.
- 2640. Arbes, S. J., Sever, M., Mehta, J., Collette, N., Thomas, B., and Zeldin, D. C. (2005). Exposure to indoor allergens in day-care facilities: results from 2 North Carolina counties. J Allergy Clin Immunol. 116[1], 133-139.
- 2641. Arbes, S. J., Jr., Gergen, P. J., Vaughn, B., and Zeldin, D. C. (2007). Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 120[5], 1139-1145.
- 2642. Asturias, J. A., Ibarrola, I., Ferrer, A., Andreu, C., Lopez-Pascual, E., Quiralte, J., Florido, F., and Martinez, A. (2005). Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J Allergy Clin Immunol. 115[6], 1210-1217.
- 2643. Barnes, S. D., Dohlman, C. H., and Durand, M. L. (2007). Fungal colonization and infection in Boston keratoprosthesis. Cornea. 26[1], 9-15.
- 2644. Bavbek, S., Erkekol, F. O., Ceter, T., Mungan, D., Ozer, F., Pinar, M., and Misirligil, Z. (2006). Sensitization to Alternaria and Cladosporium in patients with respiratory allergy and outdoor counts of mold spores in Ankara atmosphere, Turkey. J Asthma. 43[6], 421-426.
- 2645. Bonatti, H., Lass-Florl, C., Zelger, B., Lottersberger, C., Singh, N., Pruett, T. L., Margreiter, R., and Schneeberger, S. (2007). Alternaria alternata soft tissue infection in a forearm transplant recipient. Surg Infect (Larchmt.). 8[5], 539-544.
- 2647. Cardoso, S. V., Campolina, S. S., Guimaraes, A. L., Faria, P. R., da, C. Costa EM, Gomez, R. S., Rocha, A., Caligiorne, R. B., and Loyola, A. M. (2007). Oral phaeohyphomycosis. J Clin Pathol. 60[2], 204-205.
- 2649. Cooley, J. D., Wong, W. C., Jumper, C. A., and Straus, D. C. (1998). Correlation between the prevalence of certain fungi and sick building syndrome. Occup Environ Med. 55[9], 579-584.
- 2650. Douglas, R., Bruhn, M., Tan, L. W., Ooi, E., Psaltis, A., and Wormald, P. J. (2007). Response of peripheral blood lymphocytes to fungal extracts and staphylococcal superantigen B in chronic rhinosinusitis. Laryngoscope. 117[3], 411-414.
- 2658. Krysinska-Traczyk, E., Skorska, C., Prazmo, Z., Sitkowska, J., Cholewa, G., and Dutkiewicz, J. (2004). Exposure to airborne microorganisms, dust and endotoxin during flax scutching on farms. Ann Agric Environ Med. 11[2], 309-317.
- 2666. Menzies, D., Comtois, P., Pasztor, J., Nunes, F., and Hanley, J. A. (1998). Aeroallergens and work-related respiratory symptoms among office workers. J Allergy Clin Immunol. 101[1 Pt 1], 38-44.
- 2671. Podda, L., Fozza, C., Nieddu, R., Sanna, S., Paglietti, B., Vacca, A., La, Nasa G., and Longinotti, M. (2008). Breakthrough cutaneous alternariosis in a patient with acute lymphoblastic leukemia: clinical features and diagnostic issues. Leuk.Lymphoma. 49[1], 154-155.
- 2672. Rivoire, B., Attucci, S., Anthonioz, P., Carre, P., Lemarie, E., and Hazouard, E. (2001). Occupational acute lung injury due to Alternaria alternata: early stage of organic dust toxic syndrome requires no corticosteroids. Intensive Care Med. 27[7], 1236-1237.
- 2674. Romano, C., Vanzi, L., Massi, D., and Difonzo, E. M. (2005). Subcutaneous alternariosis. Mycoses. 48[6], 408-412.
- 2676. Schneider, P. B., Denk, U., Breitenbach, M., Richter, K., Schmid-Grendelmeier, P., Nobbe, S., Himly, M., Mari, A., Ebner, C., and Simon-Nobbe, B. (2006). Alternaria alternata NADP-dependent mannitol dehydrogenase is an important fungal allergen. Clin Exp Allergy. 36[12], 1513-1524.
- 2678. Shin, S. H., Ye, M. K., and Lee, Y. H. (2007). Fungus culture of the nasal secretion of chronic rhinosinusitis patients: seasonal variations in Daegu, Korea. Am J Rhinol. 21[5], 556-559.
- 2680. Skorska, C., Sitkowska, J., Krysinska-Traczyk, E., Cholewa, G., and Dutkiewicz, J. (2005). Exposure to airborne microorganisms, dust and endotoxin during processing of peppermint and chamomile herbs on farms. Ann Agric Environ Med. 12[2], 281-288.
- 2681. Sood, N., Gugnani, H. C., Guarro, J., Paliwal-Joshi, A., and Vijayan, V. K. (2007). Subcutaneous phaeohyphomycosis caused by Alternaria alternata in an immunocompetent patient. Int J Dermatol. 46[4], 412-413.
- 2683. Veer, P., Patwardhan, N. S., and Damle, A. S. (2007). Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 25[1], 53-56.
- 2684. Verma, K., Vajpayee, R. B., Titiyal, J. S., Sharma, N., and Nayak, N. (2005). Post-LASIK infectious crystalline keratopathy caused by Alternaria. Cornea. 24[8], 1018-1020.
- 2685. Vieira, R., Veloso, J., Afonso, A., and Rodrigues, A. (2006). Cutaneous alternariosis in a liver transplant recipient. Rev Iberoam.Micol. 23[2], 107-109.
- 2749. Frisvad, J. C., Filtenborg, O., and Thrane, U. (1989). Analysis and screening for mycotoxins and other secondary metabolites in fungal cultures by thin-layer chromatography and high-performance liquid chromatography. Arch Environ Contam Toxicol. 18[3], 331-335.
- 2865. Anandan, V., Nayak, V., Sundaram, S., and Srikanth, P. (2008). An association of Alternaria alternata and Scopulariopsis brevicaulis in cutaneous phaeohyphomycosis. Indian J Dermatol.Venereol.Leprol. 74[3], 244-247.
- 2919. doPico, G. A., Reddan, W. G., Chmelik, F., Peters, M. E., Reed, C. E., and Rankin, J. (1976). The value of precipitating antibodies in screening for hypersensitivity pneumonitis. Am Rev Respir Dis. 113[4], 451-455.
- 2989. Palmas, F., Cosentino, S., and Cardia, P. (1989). Fungal air-borne spores as health risk factors among workers in alimentary industries. Eur J Epidemiol. 5[2], 239-243.
- 3010. Baur, X., Behr, J., Dewair, M., Ehret, W., Fruhmann, G., Vogelmeier, C., Weiss, W., and Zinkernagel, V. (1988). Humidifier lung and humidifier fever. Lung. 166[2], 113-124.
- 3207. Mari, A., Schneider, P., Wally, V., Breitenbach, M., and Simon-Nobbe, B. (2003). Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin.Exp.Allergy. 33[10], 1429-1438.
- 3284. Hollister-Stier Laboratories. (2009). Allergenic extracts : Molds. Hollister-Stier Laboratories.
- 3285. Federal Drug Administration (FDA). (2008). Biological products deviation reporting (BPDR). Non-blood product codes. 3-29-2009.
- 3318. UniProt Consortium. (2009). Taxonomy : fungi metazoa group. Site de UniProt. 4-6-2009.
- 3483. Pohland, A. E. (1993). Mycotoxins in review. Food.Addit.Contam. 10[1], 17-28.
- 3593. Ogawa, H., Fujimura, M., Tofuku, Y., and Kitagawa, M. (2009). Eosinophilic pneumonia caused by Aspergillus niger: is oral cleansing with amphotericin B efficacious in preventing relapse of allergic pneumonitis? J Asthma. 46[1], 95-98.
- 3671. Brandt, M. E. and Warnock, D. W. (2003). Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 15 Suppl 2:36-47., 36-47.
- 3717. Van, Lancker F., Adams, A., Delmulle, B., De, Saeger S., Moretti, A., Van, Peteghem C., and De, Kimpe N. (2008). Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J Environ.Monit. 10[10], 1127-1133.
- 3729. Flannigan, B., Samson, R. A., and Miller, J. D. (2002). Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. -504 p. CRC Press.
- 3748. Lawley, R. (2009). Alternaria toxins. Site de Micotoxinas online. 7-21-2009.
- 3760. Schmechel, D., Green, B. J., Blachere, F. M., Janotka, E., and Beezhold, D. H. (2008). Analytical bias of cross-reactive polyclonal antibodies for environmental immunoassays of Alternaria alternata. J Allergy.Clin.Immunol. 121[3], 763-768.
- 3799. Goetz, D. W., Webb, E. L., Jr., Whisman, B. A., and Freeman, T. M. (1997). Aeroallergen-specific IgE changes in individuals with rapid human immunodeficiency virus disease progression. Ann.Allergy.Asthma.Immunol. 78[3], 301-306.
- 3800. Panda, A., Sharma, N., Das, G., Kumar, N., and Satpathy, G. (1997). Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea. 16[3], 295-299.
- 3802. Chang, C. Z., Hwang, S. L., and Howng, S. L. (1997). Allergical fungal sinusitis with intracranial abscess--a case report and literature review. Kaohsiung.J Med Sci. 13[11], 685-689.
- 3805. Vennewald, I., Henker, M., Klemm, E., and Seebacher, C. (1999). Fungal colonization of the paranasal sinuses. Mycoses. 42 Suppl 2:33-6., 33-36.
- 3807. Gilmour, T. K., Rytina, E., O'Connell, P. B., and Sterling, J. C. (2001). Cutaneous alternariosis in a cardiac transplant recipient. Australas.J Dermatol. 42[1], 46-49.
- 3808. Jahagirdar, B. N. and Morrison, V. A. (2002). Emerging fungal pathogens in patients with hematologic malignancies and marrow/stem-cell transplant recipients. Semin.Respir.Infect. 17[2], 113-120.
- 3809. Wheat, L. J., Goldman, M., and Sarosi, G. (2002). State-of-the-art review of pulmonary fungal infections. Semin.Respir.Infect. 17[2], 158-181.
- 3811. Zahra, L. V., Mallia, D., Hardie, J. G., Bezzina, A., and Fenech, T. (2002). Case Report. Keratomycosis due to Alternaria alternata in a diabetic patient. Mycoses. 45[11-12], 512-514.
- 3817. Kazory, A., Ducloux, D., Reboux, G., Blanc, D., Faivre, B., Chalopin, J. M., and Piarroux, R. (2004). Cutaneous Alternaria infection in renal transplant recipients: a report of two cases with an unusual mode of transmission. Transpl.Infect.Dis. 6[1], 46-49.
- 3818. Gilaberte, M., Bartralot, R., Torres, J. M., Reus, F. S., Rodriguez, V., Alomar, A., and Pujol, R. M. (2005). Cutaneous alternariosis in transplant recipients: clinicopathologic review of 9 cases. J Am.Acad.Dermatol. 52[4], 653-659.
- 3821. Torres-Rodriguez, J. M., Gonzalez, M. P., Corominas, J. M., and Pujol, R. M. (2005). Successful thermotherapy for a subcutaneous infection due to Alternaria alternata in a renal transplant recipient. Arch.Dermatol. 141[9], 1171-1173.
- 3832. Williams, C., Layton, A. M., Kerr, K., Kibbler, C., and Barton, R. C. (2008). Cutaneous infection with an Alternaria sp. in an immunocompetent host. Clin.Exp.Dermatol. 33[4], 440-442.
- 3842. Kendrick, B. and Murase, G. (2003). Anamorph-teleomorph dabase. CBS. Centraalbureau voor Schimmelcultures. 2009.
- 3847. Haubruge, E., Chasseur, C., Debouck, C., Begaux, F., Suetens, C., Mathieu, F., Michel, V., Gaspar, C., Rooze, M., Hinsenkamp, M., Gillet, P., Nolard, N., and Lognay, G. (2001). The prevalence of mycotoxins in Kashin-Beck disease. Int.Orthop. 25[3], 159-161.
- 3858. Frisvad, J. C., Andersen, B., and Thrane, U. (2008). The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol.Res. 112[Pt 2], 231-240.
- 3873. Weidenb÷rner, M. (2001). Encyclopedia of food mycotoxins.
- 3877. Hedayati, M. T., Arabzadehmoghadam, A., and Hajheydari, Z. (2009). Specific IgE against Alternaria alternata in atopic dermatitis and asthma patients. Eur.Rev.Med Pharmacol.Sci. 13[3], 187-191.
- 3878. Nasser, S. M. and Pulimood, T. B. (2009). Allergens and thunderstorm asthma. Curr.Allergy.Asthma.Rep. 9[5], 384-390.
- 3896. Bush, R. K. and Prochnau, J. J. (2004). Alternaria-induced asthma. J Allergy.Clin.Immunol. 113[2], 227-234.
- 3902. Halonen, M., Stern, D. A., Wright, A. L., Taussig, L. M., and Martinez, F. D. (1997). Alternaria as a major allergen for asthma in children raised in a desert environment. Am.J Respir.Crit.Care Med. 155[4], 1356-1361.
- 3904. Neukirch, C., Henry, C., Leynaert, B., Liard, R., Bousquet, J., and Neukirch, F. (1999). Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy.Clin.Immunol. 103[4], 709-711.
- 3905. Patel, H. J., Douglas, G. J., Herd, C. M., Spina, D., Giembycz, M. A., Barnes, P. J., Belvisi, M. G., and Page, C. P. (1999). Antigen-induced bronchial hyperresponsiveness in the rabbit is not dependent on M(2)-receptor dysfunction. Pulm.Pharmacol.Ther. 12[4], 245-255.
- 3907. Peat, J. K., Tovey, E., Mellis, C. M., Leeder, S. R., and Woolcock, A. J. (1993). Importance of house dust mite and Alternaria allergens in childhood asthma: an epidemiological study in two climatic regions of Australia. Clin.Exp.Allergy. 23[10], 812-820.
- 3910. Zureik, M., Neukirch, C., Leynaert, B., Liard, R., Bousquet, J., and Neukirch, F. (2002). Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 325[7361], 411-414.
- 3911. Simon-Nobbe, B., Probst, G., Kajava, A. V., Oberkofler, H., Susani, M., Crameri, R., Ferreira, F., Ebner, C., and Breitenbach, M. (2000). IgE-binding epitopes of enolases, a class of highly conserved fungal allergens. J Allergy.Clin.Immunol. 106[5], 887-895.
- 3912. Simon-Nobbe, B., Denk, U., Poll, V., Rid, R., and Breitenbach, M. (2008). The spectrum of fungal allergy. Int.Arch.Allergy.Immunol. 145[1], 58-86.
- 3929. Brugger, E. M., Wagner, J., Schumacher, D. M., Koch, K., Podlech, J., Metzler, M., and Lehmann, L. (2006). Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol.Lett. 164[3], 221-230.
- 3930. Morrison, V. A. and Weisdorf, D. J. (1993). Alternaria: a sinonasal pathogen of immunocompromised hosts. Clin.Infect.Dis. 16[2], 265-270.
- 3931. Morrison, V. A., Haake, R. J., and Weisdorf, D. J. (1993). The spectrum of non-Candida fungal infections following bone marrow transplantation. Medicine (Baltimore.). 72[2], 78-89.
- 3932. Schell, W. A. (2000). Unusual fungal pathogens in fungal rhinosinusitis. Otolaryngol.Clin.North.Am. 33[2], 367-373.
- 3933. Wadhwani, K. and Srivastava, A. K. (1984). Fungi from otitis media of agricultural field workers. Mycopathologia. 88[2-3], 155-159.
- 3938. Kaplan, R. L. (1982). Hypersensitivity pneumonitis due to Alternaria tenuis. Pa.Med. 85[1], 34.
- 3940. Fink, J. N., Schlueter, D. P., and Barboriak, J. J. (1973). Hypersensitivity pneumonitis due to exposure to Alternaria. Chest. 63:Suppl:49S., Suppl.
- 3942. Weiss, W. and Baur, X. (1987). [Solid phase radioimmunoassay for the quantitative determination of antigen-specific IgG-antibodies in the serum of patients with farmer's lung]. J Clin.Chem.Clin.Biochem. 25[10], 689-698.







