Phoma glomerata
Basics
Phoma is the most widely distributed genus of the Pleosporales order (or Shaeropsidales when considered as part of the Fungi Imperfecti) and the largest with some 2000 species {1878; 1056}. The taxonomy of this genus is complicated and the identification of the species is often difficult {479}. The classical generic criteria are well established but, at and below the species level, much work still remains. Many of the strains isolated from human infections have not been identified at the species level {816; 3841}. There are 3 registered species of Phoma (P. exigua, P. foveata, P. sambuci-nigrae) and 4 registered cultivars of Phoma exigua; some registered strains are yet unnamed {3318}.
Many species of Phoma adapt easily to indoor parameters and grow well on building materials. Phoma glomerata is the most common in the indoor setting; the following text will mostly address this species, but as most of the strains isolated from air samples and human infections have not been identified in terms of species level, these will be addressed herein asPhoma species.
Taxonomy
| Kingdom | Fungi | Order | Pleosporales (Sphaeropsidales) |
| Phylum | Ascomycota | Family | Pleosporaceae |
| Class | Euascomycetes | Genus | Phoma |
Phoma spp. are dematiaceous hyphomycetes anamorphs: many Phoma spp. have also known teleomorph forms included in 30 genera {3842}, the best known being Pleospora {816}, Didmyella and Leptosphaeria {3842}.
Habitat/Ecology
Phoma is an ubiquitous and widespread fungus with species found in soil and on various dead or living plant material {813; 3729; 816; 3855; 1056}; this fungus is one of the most important phytopathogen agents {1931; 1696}. In one specialised identification key, 223 taxa are said to be widespread in western Europe and also recorded in Australia, North America and Eurasia {3861}.
This fungus is also found on wood products (birch), paper, textiles (wool), leather, selected foods such as fruits and vegetables, milk products, animal and vegetable fat {989; 3729; 1056}. Phoma appears to have an affinity for lipid-containing substrates such as seeds and nuts {3965; 3967; 3966; 3968}.
The prevalence of Phoma spores in outdoor air is variable but tends to be low or very low, even if it is often reported as one of the most common and abundant fungal spores on leaf surfaces {1281; 3853}; this apparent contradiction may stem from the fact that spores contained in the fruiting bodies are not easily dispersed and that actual dispersal may be more readily accomplished by the ascopores produced by the teleomorph forms. For example, a study in Tulsa (Oklahoma) reported a prevalence of 100% in the environment and concentrations as high as 3.7 CFU/cm² on leaf surfaces of trees, representing 12.3% of total counts, followed closely by Cladosporium {1281}. Conversely, in the Netherlands, Phoma was one of the less frequent fungi in the airspora, with concentrations below 1 CFU/m3 {864}. In Thessaloniki (Greece), during 1987-2001, Phoma appeared to be the fungus with the lowest prevalence in outdoor air (0.06%) {1788}. In Saudi Arabia, Abdel-Hafez and Shoreit {930} recovered Phoma in 60% of air samples (growth on Czapek’s medium); this fungus constituted 9.5% of the total fungi of which three species were identified: P. glomerata, P. humicola and P. herbarum.
Growth requirements
Optimal temperature for growth ranges from 26 °C to 37 °C, but some species, such as P. glomerata, are not able to grow at 37 °C {989}. This hydrophilic fungus grows well on near saturated materials {3729}.
Water Activity : Aw ³ 0.90
Growth on building materials or indoor environment
In the indoor environment, Phoma sp. is common on wet and damp paints, wood, wall paper, window frames, ceiling tiles, carpets and on the reverse side of linoleum ; it is also commonly isolated from house dust {3972; 3729; 1696; 1193; 1056}. Phoma sp. may produce pink and purple spots on painted walls, because of the presence a diffusible pigment {818}. It may also grow on cement, rubber and paper. However, under controlled laboratory conditions, its growth on wet gypsum wallboard appears slow and signs of growth were not apparent before the 7th week {587}. It is also reported that Phoma growth on building materials may have little effect on indoor air quality because the spores are not readily disseminated {813}.
Phoma glomerata in particular has been reported indoors on wood, cement, oil painted surfaces and paper {3729; 1056}.
Results from indoor airborne spore prevalence studies vary greatly.
More details
In the indoor air of 68 homes in Southern California, the isolation frequency of Phoma was very low, at 1.5% {1824}. In a New York State study, culture plates were exposed for a 13-month period in work-leisure-sleep environments of 130 allergy patients; among the 20 species detected, Phoma was the 14th, with a prevalence of 14% {1821}.
In a survey of an old public swimming bath containing cloak rooms and showers in which there had been problems with leaking roofs and condensing water, surface sample results showed a massive growth of Phoma along with two other dominant species Trichoderma and Fusarium {449}. In another case of wet environment, a shower curtain heavily contaminated by a brownish-grey mat of fungal spores and mycelium revealed the presence of three species, includingPhoma violacea {1899}. The further study of 24 patients with unexplained hypersensitivity pneumonitis (HP) symptoms with allergen extracts from this fungus, showed that the sera of 4 patients, with otherwise negative serological tests, tested positive for anti-Phoma IgG.
Laboratory section
Normal laboratory precautions should be exercised in handling cultures of this species within Biosafety Level 2 practices and containment facilities.
Colony, macroscopic morphology
Colonies of Phoma grow well on general fungal media {813; 415} and are usually mature within 5 days {412}; colonies reach approximately 10 cm in diameter after 8 to 10 days on Malt Extract Agar (MEA) {989}. They are powdery, velvety or cottony, spreading greyish brown {412}. The reverse side of the colony is dark brown to olive in the center and lighter at the margin {989}; certain species produce a reddish-purple to yellowish-brown diffusible pigment {412} readily visible from the reverse {816}.
Microscopic morphology
Hyaline or brown septate hyphae (diameter 70-100 µm) bare asexual fruiting bodies (pycnidia; 200 x 50-200 mm) which are dark, round or flask shaped with openings (ostioles). The conidia, formed on conidiophores inside the pycnidia, are oval, one celled, and hyaline {989; 412; 479; 1056; 415}. The ostiolate pycnidia without septae is a distinguishing characteristic from other similar moulds {1931}. Phoma is sometimes confused with Pyrenochaeta, but the two can be distinguished on the basis of their conidium-bearing structures {479}.
Phoma glomerata can be distinguished from other common indoor Phoma species by the presence of multicellular dark chlamydospores with alternate vertical septae, somewhat resembling Alternaria spores and occasionally unicellular chlamydospores; these spores are also known as «catenulate dictyochlamydospores».
Specific metabolites
Organics compounds (including VOCs)
There are no reported volatile organic compounds (VOCs) specifically produced by Phoma spp.that would be deleterious to humans or animals.
Mycotoxins
Phoma spp. are known for the production of toxins directed at either plant or animal cells. Among these substances, some are known to be cytotoxic in animal models : cytochalasin A and B (also named phomin), deoxaphomin and proxiphomin, tenuazonic acid {3866; 1883; 3973; 3867}. According to one source, Phoma glomerata produces kojic acid and aflatoxin {989}. Phoma toxins have been isolated from contaminated grain, seeds and nuts. No data have been published on Phomatoxins produced on building materials.
More details
Phoma exiqua isolated from barley produces the following toxins: cytochalasin A and B, deoxaphomin and proxiphomin {1883}. Although uncommon, Phoma spp. have been isolated from copra, peanuts and pecans {3965; 3967; 3966; 3968}. These substrates are rich in fat and may represent a good substrate for these fungi and the production of mycotoxins, as shown in the case of tenuazonic acid detected in sunflower seed meal {3974}.
Adverse health reactions
Health risks associated with mould exposure in water damaged buildings are well established, especially for upper and lower respiratory tract symptoms. Phoma is often found growing on building materials but is one of the less prevalent airborne genera in indoor air as the spores contained in the pycnidia are not easily aerosolized. Consequently, its role is probably limited to adverse health effects attributable to indoor air quality.
Irritation and inflammation
There is no published data regarding specific substances produced by Phoma spp. that would/could cause irritation or inflammation.
More details
Generally speaking, all moulds contain substances that are irritants and promote inflammation to some degree. Some VOCs produced by moulds in the indoor setting on damp building materials are thought to contribute to various health problems, such as eye irritation, irritation of the nose and throat, and possibly lethargy and headache {594}.
Allergic reactions
Phoma is considered as a common outdoor and indoor air allergen. Phoma is reported as a fungus involved in type I allergies (hay fever, asthma) and may cause type III hypersensitivity pneumonitis {3870; 989}.
Allergic sensitisation to Phoma is more prevalent in the atopic population than its actual concentrations in outdoor air would let us predict. A Canadian study showed that in individuals with allergic rhinitis or asthma and for whom skin tests were positive to airborne fungal species, 36% were positive to Phoma glomerata, despite the fact that this species was isolated in less than 1% of outdoor and indoor air samples {1817}. In England, Buisseret found that, in patients with seasonal or summer asthma, 10% proved to be allergic to fungal spores, especially Alternaria and Phoma {3871}.
In a study of IgE antibodies in serum samples from American patients, sensitisation to Phoma was the second most common mould allergy {1893}.
Allergic reactions specifically associated with indoor exposure to Phoma have been reported.
More details
In schools with moisture problems, Taskinen et al. identified 6 children (out of 99) that tested positive to the skin prick test, including two to Phoma herbarum {720}.
In a Scandinavian study on the prevalence of specific IgE among asthmatic children previously submitted to a skin prick test, between 4 and 60% children were positive for Phoma using the RAST test, depending on their respective grouping (severity of symptoms and previous reactions to skin prick test) {1399}. In a study by Karlsson-Borga et al., an extended panel of RAST tests which combined 6 standard fungal allergens and 10 additional new mould genera, was investigated on 2 groups of adult patients (American and European) with suspected mould allergy {1893}: 73% had specific IgE antibodies to at least one of the 16 moulds tested and, in the American group, Phoma and Botrytis showed the highest prevalence, hence demonstrating the utility of an extended panel adapted to regional mycoflora.
Allergic components and mechanism
There are no published data relative to allergenic fractions of Phoma spp.
Hypersensitivity pneumonitis
Type III hypersensitivity pneumonites due to a few species of Phoma have been reported in a small number of cases.
More details
A positive serum precipitin reaction to Phoma herbarum and Aspergillus fumigatus was observed in a 72-year old woman with hypersensitivity pneumonitis {1680}.
Green (1899) reported that precipitating antibodies to Phoma violacea have been found in some patients in Sydney (Australia) suspected of having allergic interstitial pneumonitis; the species was isolated from domestic shower curtains which may provide a source of human exposure via splashing droplets {1899}.
Toxic effects (mycotoxicosis)
No human or animal confirmed mycotoxicosis associated with Phoma has been reported.
However consumption of contaminated sorghum by tenuizic acid has been proposed as a contributing factor in Onyalai {3860}.
More details
Onyalai is a form of thrombocytopenic purpura disease due to a nutritional disorder occurring in blacks in central southern Africa. Onyalai is a disease of unknown aetiology. Defective nutrition may be the cause. One hypothesis is that the mycotoxin, possibly acting as a hapten, is responsible for this form of thrombocytopenia. The possible aetiological role of mycotoxins from contaminated millet, sorghum and/or maize requires further investigation {3859}.
Infections and colonisations
Phoma species are rarely pathogenic to humans and animals; until 2001, only 13 cases of human Phoma infections, mainly involving skin and subcutaneous tissues, have been reported in the literature {1878}. Phoma usually causes superficial skin lesions, which are typically erythematous, crusted and itchy {1885}. However, opportunistic infections (phaeohyphomycosis) leading to respiratory, subcutaneous and cornea (keratitis) infections have also been occasionally reported {1879; 813; 818; 816; 1933}.
During the past 30 years, Phoma spp. have been repeatedly isolated from clinical material and hence have gradually assumed an increasing importance as an opportunist pathogen in humans {1931; 1892}.
Individuals with a weakened immune system or suffering a trauma are particularly at risk {1932; 1696; 816}; reports of cutaneous infections caused by this fungus have occurred in patients with loss of local skin defences or chronically immunosuppressed {1885}.
Phoma infections through an inhalation route appear extremely rare.
More details
Skin and related tissue infections due to Phoma seem to occur solely on exposed areas (hands, feet and face), suggesting that they are the result of minor local inoculations with the contaminated source; typically, the fungus is introduced into the skin by trauma {1878}. For example, a phaeohyphomycosis case was reported in a 45-year old man with no known immunological defect; however, his hands frequently sustained minor lacerations and abrasions with frequent contact with soil and plant products {1890} . In India, a young man was reported with erythematous papuloviscular lesions on the face, neck and hands; another male patient had round spots over his entire face; in both cases, Phoma sorghina was reported as the causative agent {1892}.
A pharmaceutically induced weakening of the immune system may explain certain Phoma infections. Phoma minutella was identified as the causative agent of a subcutaneous phaeohyphomycosis on the foot of a farmer undergoing corticosteroid therapy for myasthenia gravis {1931}. Another case of subcutaneous phaeohyphomycosis (nodular erythematous-violaceous skin lesions in the dorsum of the right hand) was reported in a man presenting pulmonary sarcoidosis and submitted to corticosteroid treatment {1933}. Everett et al. {1885} reported an aggressive and deeply invasive Phoma infection of the hand in a chronically immunosuppressed 50-year old renal transplant patient who required surgical debridement; however, no history of previous trauma was reported. A similar case was also reported in a renal allograft recipient, agriculturist by profession, who developed localized subcutaneous infection in both forearms following trauma one year after a renal transplant {1932}.
A rare case of keratitis was reported in a 72-year old man after a recent history of eye trauma {1878}.
In 2006, a fatal case of lung infection due to Phoma exigua was reported in a patient with acute myeloid leukaemia and diabetes {1879}; this was only the second case of lung infection by Phoma reported in the literature {3868}.
Virulence factors
No particular virulence factors have been reported for Phoma spp.
Specific settings
Nosocomial infections
There are no published cases of nosocomial Phoma infection.
Occupational diseases
There are no published cases of Phoma related diseases strictly associated with the occupational setting. However, one published study recounts the occurrence of many symptoms in workers exposed to Phoma and other fungi in a contaminated building.
More details
In this particular case, employees exposed to fungi in a heavily contaminated public bath where Phoma was among the three dominating species, experienced, among others, many allergenic symptoms including eye, nose and throat irritation, as well as headaches and dizziness; all of the symptoms were linked to mould exposure {449}. The first mitigation procedures eradicated most visible signs of moulds and resulted in a decreased number of symptoms. The second series of decontamination procedures sufficiently cleansed the building, yielding a subsequent decrease in the rate of symptoms in exposed employees and a return to normal peak-flow variability levels.
Diagnostic tools
Histopathology
The histological presentation of subcutaneous infections due to Phoma sp. is typically that of a deep sited phaeohyphomycosis. In a particularly aggressive and deeply invasive Phoma infection, histological sections obtained from the synovium of the fourth and fifth dorsal hand compartments revealed invasive hyphal elements. Detailed examination with Grocott-Gomori methenamine-silver staining revealed branching filaments and pycnidia {1885}. In one case of corneal infection, histopathological examination revealed round spherules of variable diameter (5-30 µm) admixed with septate hyphae at the edges of the perforated cornea {1878}. In the case of a fatal Phoma exigua lung infection in a patient with acute myeloid leukaemia and diabetes, a large mass was examined in which haematoxylin and eosin stained preparations showed broad septate thick-walled hyphae in the lung tissue {1879} .
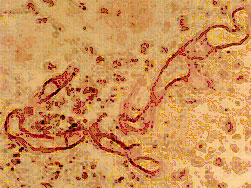
A lung section showing large hyphal elements {1879}.
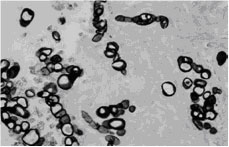

A keratotomy specimen showing a detached epithelium (top left). Large spherules of the fungus are observed in the superficial stroma (Periodic-acid-Schiff x 40) {1879}. Spherules admixed with short septate hyphae are seen in the deep corneal stroma adjacent to the site of a perforation (Grocott-methenamine silver x 128) {1878}.
Immunodiagnosis
The most common commercially available allergen preparations for use in vivo and/or in vitro are Phoma herbarum for in vivo testing (skin tests) and Phoma betae (m13) for in vitro testing {3284; 581}.
The Phoma allergen extracts listed by the FDA’s Non-Blood Product Codes registry are as follows {3285}:
- GK45 - Phoma betae
- GK46 - Phoma destructiva
- GK47 - Phoma herbarum
- GK48 - Phoma hibernica
- GK49 - Phoma sp.
| Test | IgE | IgG | Antigens | Other |
|---|---|---|---|---|
| Skin Tests | X | |||
| RAST-IgE | X | |||
| RAST-IgG | ||||
| ELISA-ELIFA | ||||
| Immunodiffusion | ||||
| Immunofluorescence | ||||
| Complement fixation | ||||
| PCR | Experimental | |||
| Other |
Bibliography
- 412. Larone, D H. (1987). Medically important fungi. A guide to identification. 2nd edition, -230 p. New York - Amsterdam - London, Elsevier Science Publishing Co., Inc.
- 415. St-Germain, G and Summerbell, R. (1996). Champignons filamenteux d'intérêt médical. Caractéristiques et idenfication. -314 p. Belmont, Star Publishing Company.
- 449. Ebbehoj, N. E., Hansen, M. O., Sigsgaard, T., and Larsen, L. (2002). Building-related symptoms and molds: a two-step intervention study. Indoor Air. 12[4], 273-277.
- 479. Malloch, D. (1997). Moulds: isolation, cultivation, identification. http://www.botany.utoronto.ca/ResearchLabs/MallochLab/ . University of Toronto. 6-22-0000.
- 581. Pharmacia Diagnostics AB. (2007). Allergy & autoimmunity. Diagnostics product catalogue 2007. internet , 1-48. Pharmacia.
- 587. Krause, M., Geer, W., Swenson, L., Fallah, P., and Robbins, C. (2006). Controlled study of mold growth and cleaning procedure on treated and untreated wet gypsum wallboard in an indoor environment. J Occup.Environ Hyg. 3[8], 435-441.
- 594. Claeson, A. S., Levin, J. O., Blomquist, G., and Sunesson, A. L. (2002). Volatile metabolites from microorganisms grown on humid building materials and synthetic media. J Environ Monit. 4[5], 667-672.
- 720. Taskinen, T., Meklin, T., Nousiainen, M., Husman, T., Nevalainen, A., and Korppi, M. (1997). Moisture and mould problems in schools and respiratory manifestations in schoolchildren: clinical and skin test findings. Acta Paediatr. 86[11], 1181-1187.
- 813. EMLAB. (2007). Environmental Microbiology Laboratory, Inc. (EMLab): An index of some commonly encountered fungal genera.
- 816. Patterson, T. F., McGinnis, M. R., and ed. (2009). The fungi :description. Site Doctor Fungus . Mycoses Study Group.
- 818. Friedman, D. (2007). Mold atlas of indoor clinical mold, pathogens, allergens and other indoor particles - medical health effects of mold, house dust, fiberglass, animal dander, insect fragments, etc. http://www.inspect-ny.com/mold/moldatlas.htm .
- 864. Beaumont, F., Kauffman, H. F., de Monchy, J. G., Sluiter, H. J., and de Vries, K. (1985). Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. II. Comparison of aerobiological data and skin tests with mould extracts in an asthmatic population. Allergy. 40[3], 181-186.
- 930. Abdel-Hafez, S. I. and Shoreit, A. A. (1985). Mycotoxins producing fungi and mycoflora of air-dust from Taif, Saudi Arabia. Mycopathologia. 92[2], 65-71.
- 989. Centre de recherche sur la conservation des documents graphiques. (2007). Moisissures et biens culturels. Ministère de la culture et de la Communication, France .
- 1056. Samson, RA, Hoekstra, ES, and Frisvad, JC. (2004). Introduction to food and airbone fungi. 7th, -389 p. Baarn, Centralalbureau voor Schimmellcultures, Institute of the Royal Netherlands Academy of Arts and Sciences.
- 1193. Nolard, N., Beguin, H., and Chasseur, C. (2001). [Mold allergy: 25 years of indoor and outdoor studies in Belgium]. Allerg.Immunol.(Paris). 33[2], 101-102.
- 1281. Levetin, E. and Dorsey, K. (2006). Contribution of leaf surface fungi to the air spora. Aerobiologia 22[1], 3-12.
- 1399. Koivikko, A., Viander, M., and Lanner, A. (1991). Use of the extended Phadebas RAST panel in the diagnosis of mould allergy in asthmatic children. Allergy. 46[2], 85-91.
- 1680. Moran, J. V., Greenberger, P. A., and Patterson, R. (2002). Long-term evaluation of hypersensitivity pneumonitis: a case study follow-up and literature review. Allergy Asthma Proc. 23[4], 265-270.
- 1696. Mold & Bacteria Consulting Laboratories (MBL) Inc. (2005). Indoor mould: do not panic! Indoor Mold and Bacteria [June, 30].
- 1788. Gioulekas, D., Damialis, A., Papakosta, D., Spieksma, F., Giouleka, P., and Patakas, D. (2004). Allergenic fungi spore records (15 years) and sensitization in patients with respiratory allergy in Thessaloniki-Greece. J Investig.Allergol.Clin Immunol. 14[3], 225-231.
- 1817. Tarlo, S. M., Fradkin, A., and Tobin, R. S. (1988). Skin testing with extracts of fungal species derived from the homes of allergy clinic patients in Toronto, Canada. Clin Allergy. 18[1], 45-52.
- 1821. Rogers, S. A. (1984). A 13-month work-leisure-sleep environment fungal survey. Ann Allergy. 52[5], 338-341.
- 1824. Kozak, P. P., Gallup, J., Cummins, L. H., and Gillman, S. A. (1979). Factors of importance in determining the prevalence of indoor molds. Ann Allergy. 43[2], 88-94.
- 1878. Rishi, K. and Font, R. L. (2003). Keratitis caused by an unusual fungus, Phoma species. Cornea. 22[2], 166-168.
- 1879. Balis, E., Velegraki, A., Fragou, A., Pefanis, A., Kalabokas, T., and Mountokalakis, T. (2006). Lung mass caused by Phoma exigua. Scand.J Infect Dis. 38[6-7], 552-555.
- 1883. Lugauskas, A., Raila, A., Railiene, M., and Raudoniene, V. (2006). Toxic micromycetes in grain raw material during its processing. Ann Agric Environ Med. 13[1], 147-161.
- 1885. Everett, J. E., Busick, N. P., Sielaff, T., Wahoff, D. C., and Dunn, D. L. (2003). A deeply invasive Phoma species infection in a renal transplant recipient. Transplant.Proc. 35[4], 1387-1389.
- 1890. Hirsh, A. H. and Schiff, T. A. (1996). Subcutaneous phaeohyphomycosis caused by an unusual pathogen: Phoma species. J Am Acad Dermatol. 34[4], 679-680.
- 1892. Rai, M. K. (1989). Phoma sorghina infection in human being. Mycopathologia. 105[3], 167-170.
- 1893. Karlsson-Borga, A., Jonsson, P., and Rolfsen, W. (1989). Specific IgE antibodies to 16 widespread mold genera in patients with suspected mold allergy. Ann Allergy. 63[6 Pt 1], 521-526.
- 1899. Green, W. F. (1972). Precipitins against a fungus, Phoma violacea, isolated from a mouldy shower curtain in sera from patients with suspected allergic interstitial pneumonitis. Med J Aust. 1[14], 696-698.
- 1931. Baker, J. G., Salkin, I. F., Forgacs, P., Haines, J. H., and Kemna, M. E. (1987). First report of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella. J Clin Microbiol. 25[12], 2395-2397.
- 1932. Kalyani, M., Ranjitham, M., Sekar, U., Indhumathy, and Soundarajan, P. (2006). Subcutaneous infection by Phoma species in a renal allograft recipient - A case report. Indian Journal for the Practising Doctor 3[1].
- 1933. Zaitz, C., Campbell, I., Moraes, J. R., Moraes, M. E., Gouvea, C., Romero, M., Gouvea, N., Sadahiro, A., Chamone, D., and Dorlhiac, P. (1996). HLA-associated susceptibility to chronic onychomycosis in Brazilian Ashkenazic Jews. Int J Dermatol. 35[9], 681-682.
- 3147. Torres-Rodriguez, J. M., Lowinger, M., Corominas, J. M., Madrenys, N., and Saballs, P. (1993). Renal infection due to Absidia corymbifera in an AIDS patient. Mycoses. 36[7-8], 255-258.
- 3284. Hollister-Stier Laboratories. (2009). Allergenic extracts : Molds. Hollister-Stier Laboratories .
- 3285. Federal Drug Administration (FDA). (2008). Biological products deviation reporting (BPDR). Non-blood product codes. 3-29-2009.
- 3318. UniProt Consortium. (2009). Taxonomy : fungi metazoa group. Site de UniProt . 4-6-2009.
- 3729. Flannigan, B., Samson, R. A., and Miller, J. D. (2002). Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. -504 p. CRC Press.
- 3841. Van der AA, H. A., Noordeloos, M. E., and de Gruyter, J. (1990). Species concepts in some larger genera of the Coelomycetes. [32]. Studies in Mycology No. 32.
- 3842. Kendrick, B. and Murase, G. (2003). Anamorph-teleomorph dabase. CBS. Centraalbureau voor Schimmelcultures. 2009.
- 3853. Osono, T. (2006). Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can.J Microbiol. 52[8], 701-716.
- 3855. Rai, M., Deshmukh, P., Gade, A., Ingle, A., Kovics, G. J., and Irinyi, L. (2009). Phoma Saccardo: Distribution, secondary metabolite production and biotechnological applications. Crit.Rev.Microbiol. 35[3], 182-196.
- 3859. Hesseling, P. B. (1992). Onyalai. Baillieres.Clin.Haematol. 5[2], 457-473.
- 3860. Rabie, C. J., van Rensburg, S. J., Van Der Watt, J. J., and Lubben, A. (1975). Onyalai--the possible involvement of a mycotoxin produced by Phoma sorghina in the aetiology. S.Afr.Med J. %20;49[40], 1647-1650.
- 3861. Boerema, G. H. (2004). Phoma Identification Manual: Differentiation of Specific and Infra-specific Taxa in Culture.
- 3866. Cole, R. J., Jarvis, B. B., and Schweikert, M. A. (2003). Handbook of secondary fungal metabolites. [3]. Amsterdam, Academic Press.
- 3867. Weidenb÷rner, M. (2001). Pine nuts: the mycobiota and potential mycotoxins. Can.J.Microbiol. 47, 460-463.
- 3868. Morris, J. T., Beckius, M. L., Jeffery, B. S., Longfeld, R. N., Heaven, R. F., and Baker, W. J. (1995). Lung mass caused by Phoma species. Infectious Diseases in Clinical Practice 4[1], 58-59.
- 3870. Burge, H (1995). Bioaerosols. 319 p.
- 3871. Buisseret, P. D. (1976). Seasonal allergic symptoms due to fungal spores. Br.Med J. 2[6034], 507-508.
- 3965. Huang, L. H. and Hanlin, R. T. (1975). Fungi occurring in freshly harvested and in-market pecans. Mycologia. 67[4], 689-700.
- 3966. Pitt, J. I., Hocking, A. D., Bhudhasamai, K., Miscamble, B. F., Wheeler, K. A., and Tanboon-Ek, P. (1993). The normal mycoflora of commodities from Thailand. 1. Nuts and oilseeds. Int.J Food.Microbiol. 20[4], 211-226.
- 3967. Joffe, A. Z. (1969). The mycoflora of fresh and stored groundnut kernels in Israel. Mycopathol.Mycol.Appl. 39, 255-264.
- 3968. Weidenbörner, M. and Hindorf, H. (1989). Fungi isolated from protein enriched seeds and pods with special emphasis on the genus Aspergillus. Sci & Technol. 17[2], 383-390.
- 3972. Beguin, H. and Nolard, N. (1996). Prevalence of fungi in carpeted floor environment: analysis of dust samples from living-rooms, bedrooms, offices and school classrooms. Aerobiologia 12, 113-120.
- 3973. Visconti, A., Logrieco, A., Vurro, M., and Bottalico, A. (1987). Tenuazonic acid in blackmold tomatoes: occurrence, production by associated Alternaria species, and phytotoxic properties. Phytopatol.Mediterran. 26, 125-128.