Aspergillus fumigatus
Basics
A. fumigatus has been associated with each and every type of health problems linked to environmental moulds: irritation and inflammation, allergy, asthma, pneumonitis, toxic effects as well as a wide range of infections. Infections have been reported affecting every anatomical site and type of tissue except nails. It is the main agent of aspergillosis in patients with impaired natural immunity. Exposure can be mostly through contaminated outdoor or indoor air, decaying plant material including compost and waste water, contaminated grain, crops and wood.
Taxonomy
| Kingdom | Fungi | Order | Eurotiales |
| Phylum | Ascomycota | Family | Trichocomaceae |
| Class | Eurotiomycetes | Genus | Aspergillus |
There are over 200 named species of Aspergillus {3318}.The taxonomic fungal database administrated by the International Mycological Association (MycoBank) has 799 registered species and strains of Aspergillus {3971}. Aspergillus fumigatus was first officially described 1863 and MycoBank reports up to 17 varieties of this species. This anamorph fungus has no reported teleomorph stage.
Habitat/Ecology
This fungus is a world-wide saprophyte, isolated from soil, decaying plant materials, compost, wood chips, hay and crops and stored grains {1089; 989; 1052}. This species grows well at temperatures up to 45°C, or even higher, and represents one of the most common microorganisms present in peat, compost and other organic material undergoing decomposition at high temperatures.
More details
A. fumigatus is often found in bird nesting environments: plant debris from birds' nests, birds' roosts and feathers, birds' droppings and chicken pens {3971}. This fungus is common in wet environments, fresh water, sea water and marshy lands, sand beaches, saline soils and mangrove swamps. It has also been isolated from sewage, activated sludge and slime of a paper mill {3971}.
A. fumigatus also grows well on indoor organic substrates, such as house dust, gypsum board and other cellulose building materials, in humidifier and air-conditioning systems, air ducts and filters, and also on household articles made of linen, leather and paper {989; 413}. The mode of dissemination of dry spore is by wind.
Growth requirements
A. fumigatus is a thermotolerant-thermophilic fungus, able to grow between 12 and 57 °C, (mean 37 - 43 °C); maximum growth in vitro when incubated at 37 °C, at pH 3-8. Its minimum available water requirements (Aw) is between 0.82-0.97 {989; 3729; 413}, making this fungus a secondary coloniser. Viability is maintained at temperatures up to 70 ° {812}, and this fungus can sustain pasteurization temperatures (63 °C) for about 25 minutes {989}.
Water Activity : Aw = 0,85 – 0,94
Growth on building materials or indoor environment
A. fumigatus readily grows in the indoor environment on dampened building materials (plasterboard, wood, chipboard, ceiling tiles, cardboard and insulation material) usually producing a light to medium growth, grey to greyish-green {595}. A. fumigatus has been also found in wet spray-applied cellulose insulation (WSACI) {670}.
A. fumigatus grows often well on indoor organic substrates such as house dust and other cellulose containing materials, in humidifier, air-conditioning systems and air ducts and filters, as well as on household articles made of linen, leather and paper {989; 413}.
Laboratory section
Normal laboratory precautions should be exercised in handling cultures of this species within Biosafety Level 2 practices and containment facilities.
Colony, macroscopic morphology
A. fumigatus grows fast at 25°C on MEA or PDA ; colonies attain a diameter of 2.5 cm within 5 days. Single point inoculum colony grown at 37°C, will be 4.0 - 6.0 cm in diameter in 7 days.
Colonies are flat or lightly wrinkled, low growing, dense with bluish green aerial mycelium. Mature colonies have a cottony surface; a purple pigment may diffuse in the agar; the reverse side is purple with a white margin {724}.
At low magnification, surface conidial heads appear, at first, with well defined radiate heads; later, at maturity, the heads are compact, long and tightly columnar.
Microscopic morphology
The main structure of the conidial head is the conidiophores, which is an elongated cell (stipe), 200-400 µm long, sometimes sinuous, with colourless, thin, smooth walls. This cell enlarges gradually into pyriform often greenish clavate vesicle (20-30 µm diam) bearing densely packed phialides (6-8 x 2-3 µm). The lateral phialides are bent upwards so that the tips are approximately parallel to the stipe axis, thus all phialides point in same direction. Conidia are spherical (2.5-3.0 µm diameter) with a finely roughened wall. Occasionally, chains of conidia may be borne directly on the vesicle {372; 2943; 991; 724}.
Specific metabolites
Organics compounds (including VOCs)
A number of organic compounds, including volatile organic coumponds (VOCs), have been identified in indoor air in damp buildings contaminated by fungi; some of these compounds are thought to contribute to various indoor air problems, However, most of the identified metabolites are non-reactive and found in low concentrations in indoor air {594}.
Aspergillus fumigatus is however the most important species in Aspergillus genus causing health problems. This species has been reported to produce a large number of substances, including secondary metabolites, acids, proteins and extracellular enzymes. At least 226 potentially bioactive metabolites have been reported from A. fumigatus {4120}. Nine “unique microbial volatile organic compounds” (umVOCs) produced by Aspergillus species cultivated on gypsum board can be found. Among them, methyl-butanol, methyl-propanol, heptanone, hexanone and octanone have been identified {598}.
Mycotoxins
Aspergillus fumigatus is known to produce mycotoxins, several being harmful to humans or animals. Depending on the nature of the substrate, environmental conditions and the strain, A. fumigatus, may produce different mycotoxins: fumagillines, fumigacine, fumigaclavines, fumigatin , fumigatotoxin , fumitoxin, fumitremorgin {989}; A. fumigatus also produces gliotoxin, a very potent immunomodulatry toxin.
A few mycotoxins such as festuclavine, chanoclavine, fumitremorgins and fumitoxins have been studied; some of them cause death of chickens and tremors (shivering) in various animals {777; 779}.
More details
Gliotoxin may be produced on spruce wood, gypsum board or chipboard. In experimentally mould-damaged building materials, the levels detected were from 1 to 40 ng (nanograms) of gliotoxin per cm²; according to these results, the amount of gliotoxin detected from an area of 100 cm² (10 cm X 10 cm) of gypsum board could lower the ciliary beating frequency of the respiratory mucosa {595}.
Other secondary metabolites may be deleterious to humans and animals. Among them are : brevianamide f phenyltransferase ,canescin, dechloronidulin, isotryptoquivaline, methyl-sulochrin, norisotryptoquivaline, pyripropene A, Sphingofungin A, spinulosin and tripacidin {812}.
Indoors, A. fumigatus can produce toxins on common materials such as latex painted surfaces. This fungus accumulates ergot alkaloids in a respirable form. At least four ergot alkaloids were found associated with A. fumigatus: fumigaclavine C, festuclavine, fumigaclavine A, and fumigaclavine B (in order of abundance). Under environmentally relevant conditions, the total mass of ergot alkaloids can often constitute more than 1% of the mass of the fungus. The greatest quantities were measured when the fungus was cultured on latex paint or maize seedlings {4110; 765}.
Studies performed on infection isolates suggest that endogen production of these toxins in human lung infections and lung aspergilloma can contribute to the pathology {4160}.
One study included 11 adult patients (seven men and four women) who had been surgically treated for pulmonary aspergilloma. Mycological analysis was positive for Aspergillus genus in five samples of surgically removed tissue. A. fumigatus was isolated in three and A. versicolor in two samples. A. fumigatus was found to produce aflatoxin B1 (AFB), and aflatoxin G1. A. versicolor produced AFB1 and sterigmatocystin. None of the isolated Aspergillus produced aflatoxin G2 or ochratoxin A. It is important to consider that the production of mycotoxins in vitro does not reflect what these fungi may produce in human tissue {4160}.
Adverse health reactions
Health risks associated with exposure to Aspergillus fumigatus are well established. Most important, A. fumigatus is the main agent of aspergillosis in patients with impaired natural immunity. This species causes a typical inhalation mycosis, with colonization followed, in some cases, by invasion and occasionally allergic reactions. Other types of infections may occur in the immunocompromised host but hardly ever in the general population.
As with other moulds, A. fumigatus may be responsible for other health problems. However, the most serious diseases caused by this fungus are the acute and chronic infections of the respiratory system (aspergillosis, aspergilloma) of man and animals {4311}.
This fungus is the cause of disease in cattle (mycotic abortion) and horses. Aspergillosis is a major cause of morbidity and mortality in certain species of birds, whether in captive or free-ranging environments and may cause the death of entire flocks kept in captivity.
Irritation and inflammation
Generally speaking, all moulds contain common substances that are irritants and promote inflammation to some degree. Many non-specific respiratory symptoms can arise from an Aspergillus exposure, including rhinitis, rising phlegm, night cough, and hoarseness; some eye and skin irritation have also been reported {2740; 142; 165; 2553; 2111; 595; 766; 946; 355; 154; 260}.
Allergic reactions
Aspergillus fumigatus is one of the important fungi responsible for inducing respiratory allergy disorders and allergic reactions in some people {1982; 1420; 1399; 3047; 3840; 2019; 3581; 3639}.
Aspergillus fumigatus is also known to produce polypeptide allergens responsible for rhinitis and asthma {675}, a Type-I allergic reaction (IgE mediated) to the spores and fragments of the mould.
A. fumigatus can cause an allergic disease associated with long standing symptoms of a runny blocked up nose leading to nasal polyps {931}. A. fumigatus may cause allergic rhinitis and asthma without invading the tissues but in some cases these allergic reactions can result from a growth in the pulmonary secretions of the bronchi; this condition is called allergic bronchopulmonary aspergillosis (ABPA).
More details
Allergic bronchopulmonary aspergillosis (ABPA).
ABPA is an infrequent complication of asthma or cystic fibrosis. Recognition of this disorder is important to avoid progression of bronchiectasis and lung parenchymal damage. Patients with this condition usually have specific antibodies (IgE) and, in some cases, a scattered pulmonary infiltrates can be observed. Up to 5% of adult asthmatics might get this at some time during their lives. This disease is common in cystic fibrosis patients as they reach adolescence and adulthood. Symptoms are similar to those of asthma: intermittent episodes of feeling unwell, coughing and wheezing {3887}. Some patients cough up brown-coloured plugs of mucus {812}. Most patients with ABPA will be diagnosed based on clinical, radiographic, and laboratory criteria {226; 822; 275; 406; 174; 206; 233; 376}.
Allergic components and mechanism
More than 40 IgE binding components from A. fumigatus have been studied either in human subjects or in the laboratory {3912}.
Over 20 genes encoding A. fumigatus antigens have been cloned and the proteins expressed. Among these allergens, Asp f 1, f 2, f 3, f 4, and f 6 showed strong but diverse IgE binding properties with sera from groups of patients {186}. The most important allergen fraction seems to be ASP-f-1: it is recognised by 85% of ABPA patients and most skin-test positive patients {3912}.
More details
Results currently available suggest that Asp f 2, f 3, and f 6 together react with IgE from patients with asthma and allergic bronchopulmonary aspergillosis (ABPA). The molecular structure of allergens also plays a major role in the immunological response in the allergic patients. Antigens can be engineered with less or more binding potential with IgE, and such antigens may have significant roles as specific reagents or as immunomodulators.
Immunological reactions to both allergens and toxins have been linked to ABPA {1287; 4144; 192; 174; 560}. «Asp f I» has been identified as a major allergen of Aspergillus fumigatus and shows homology with some cytotoxins. In one study, cytotoxins from Aspergillus species were compared for their reactivity with immunoglobulin E (IgE) and immunoglobulin G (IgG) antibodies in the serum of patients with ABPA {4144}. Elevated levels of IgE and IgG antibodies to all A. fumigatusantigens and to ribotoxins were demonstrated in a majority of ABPA patients, as compared with allergic patients with asthma and normal subjects. These results suggest that cytotoxins play a major role in the pathogenesis of allergic bronchopulmonary aspergillosis.
Hypersensitivity pneumonitis
Hypersensitivity pneumonitis (HP) or extrinsic allergic alveolitis caused by A. fumigatus is one of the best known hypersensitivity disease resulting of professional exposure; when handling mouldy hay it is called «Farmer’s lung disease» or FLD {227; 854; 271; 1103; 3670; 1091; 2245}.
Many other similar syndromes are associated with exposure to A. fumigatus from organic products such as wood chips (wood-trimmers’ disease in sawmill workers) {265; 777; 854; 231} and esparto grass contained in some plaster work (esparto-induced hypersensitivity pneumonitis or plasterer’s lung) {293; 208; 189; 246; 4168}; HP due to A. fumigatus has also been associated with indoor exposures to contaminated building materials {846} and stagnant contaminated water {230}.
Symptoms reported range from cough, dyspnoea, wheezing, and chest tightness to less specific symptoms like fatigue, myalgias and «sick feeling», {1100}. Farmer’s lung (FLD) and other forms of HP can lead to interstitial fibrosis. The progression to lung fibrosis is extremely variable; some, subjects with HP will completely recover after one or multiple attacks of acute disease, while others will have residual functional alterations. Some subjects may develop progressive lung fibrosis without ever having had the acute phase, of the disease {979}. In rare cases, patients have typical systemic manifestations of acute farmer's lung, but without the lung involvement required to confirm that disease {981}.
Toxic effects (mycotoxicosis)
Mycotoxins derived from Aspergillus spp. can be encountered both in domestic and occupational environments, and the exposure may lead to severe health hazards. Exposure to mycotoxins may occur via ingestion, inhalation or direct contact to skin and mucosa. Acute and chronic disorders, irritation, systemic reactions and even cancer may develop after the exposure to these toxins. Some mycotoxins act as immunosuppressants which may be in association with an increased prevalence of repeated infections found among the inhabitants of buildings with moisture problems {3437}.
Most reported cases of mycotoxicoses are of food borne origin. In humans, there are very few documented cases of inhaled mycotoxicoses. In humans, no cases of mycotoxicosis in the strict sense (with a specific clinical definition) due to A. fumigatus mycotoxins has been reported. The toxins of A. fumigatus however are said to contribute to other mould related health problems and to the pathogenicity of this fungus. As A. fumigatus has the capacity of producing many harmful toxins, we cannot discard the pathogenic potential of these metabolites.
More details
Most studies on Aspergillus fumigatus toxic effects focus primarily on tremorgenic mycotoxins and on gliotoxin {299}. It is reported that, experimentally, some A. fumigatus strains are able to produce toxins which can induce very strong tremorgenic reactions. Although the same toxins were identified in strains isolated from sawmills in Sweden and could induce a possible toxic health problems in these workplaces, no such mycotoxicosis cases have been yet reported in wood-trimmers workers {777; 779}.
It has been shown that gliotoxin can be produced indoors on spruce wood, gypsum board or chipboard. In the experimentally mould-contaminated building materials. The amount of gliotoxin detected, for example, from an area of 100 cm² of gypsum board, could lower the ciliary beating frequency of the respiratory mucosa, a mechanism implicated in the cytotoxicity of gliotoxin {595}.
Other studies of experimental mycotoxicosis in mice, chicks and pigs have produced the appearance of perirenal oedemain pigs {1243} with toxins from A. fumigatus.
Verruculogen associated with Aspergillus fumigates modifies the electrophysiological properties of human nasal epithelial cells; one study demonstrated that verruculogen could be produced by a collection of 67 A. fumigatus isolates and could play a role in the colonization of the respiratory tract by its conidia {4140}. An other study showed that this toxin could be produced by both airborne and clinical isolates of Aspergillus fumigatus {300}. In both groups, high percentage of verruculogen-producing strains was noticed (84% of airborne and 91% of clinical isolates). Verruculogen production was not significantly different in the groups of airborne and clinical isolates.
One study suggests a link between the indoor environmental exposure to toxic moulds (A. fumigatus being one of them) with cases of acoustic mycotic neuroma in adolescents {711}; the link remains however to be studied.
Infections and colonisations
Aspergillus fumigatus is increasingly recognized as an important opportunistic pathogen in severely immunocompromised patients. Aspergillus species have emerged as the most common infectious cause of pneumonic mortality in bone marrow/stem cell transplant recipients {2123}. These infections are difficult to diagnose antemortem and typically have a fatal outcome {4134}.
The primary site of colonisation and infection is the respiratory tract; the severe disseminated forms of infection may progress to the brain, bone and circulatory system. Other infection sites include sinuses, ears, eyes, kidneys and gastrointestinal tract. {4270; 812; 415}.
In the normal host, non-respiratory infections are infrequent because the healthy individual has a naturally high resistance to infection {812}. Thus, A. fumigatus species is usually isolated from immunocompromised, acute leukemia, transplant recipients, patients with autoimmune diseases and HIV patients {299}. Those receiving antimicrobial agents or steroid treatment or people infected with certain microorganisms, are also at risk {406; 605}.
Populations at risk for severe infections are neutropenic patients {683; 54; 363}, transplant patients {517; 685; 258; 354; 491; 990; 684}, patients under chemotherapy or steroids {338; 512} and those with chronic granulomatous disease {228} ; systemic cases have also been occasionally reported in patients without apparent underlying disease {4312}.
More details
Aspergilloma and chronic pulmonary colonisation
A. fumigatus can cause a disease without penetrating the organs. The fungus grows within a cavity of the lung, which could have been previously formed during an illness such as tuberculosis or sarcoïdosis (any lung disease which causes cavities can leave a person open to developing an aspergilloma). The spores penetrate the cavity and germinate, forming a fungal ball within the cavity. The fungus secretes toxic and allergenic products. The person affected may have no symptoms, especially early on. Weight loss, chronic cough and feeling rundown and tired are later common symptoms. Coughing of blood (haemoptysis) can occur in up to 50-80% of affected people. The diagnosis is made by X-rays, scans of lungs and blood tests {812}.
Aspergillomas or fungal balls can form elsewhere than in the lungs, such as in the sinuses. In subjects with normal immune systems, stuffiness of the nose, sinus pain, chronic headache or discomfort in the face is common. In these cases, drainage of the sinus by surgery usually cures the problem unless the Aspergillus has entered deep inside the skull. Then antifungal drugs and surgery are usually successful.
Pulmonary and invasive aspergillosis
Although sites of infections other than the respiratory tract have been described (kidneys, eye, ears, sinuses, and gastrointestinal tract), they seldom happen in immunocompetent individuals. When patients have impaired immune systems, Aspergillus sinusitis is more serious. In these cases, the sinusitis is a form of invasive aspergillosis. The symptoms include fever, facial pain, nasal discharge and headaches. The diagnosis is made by finding the fungus in fluid or tissue from the sinuses and with scans {812}.
In people with particularly poor immune systems, the fungus can transfer from the lung through the blood stream to the brain or to other organs, including the eyes, the heart, the kidneys and the skin. However, occasionally, the resulting disseminated infection to the skin enables the diagnosis to be made earlier and treatment to be started sooner {1090; 812}.
People with invasive aspergillosis usually have a fever and pulmonary symptoms such as cough, chest pain or discomfort or breathlessness, which do not respond to standard antibiotic therapy. X-rays and scans are usually abnormal and help to localise the disease. Bronchoscopy (inspection of the inside of the lung with a small tube inserted via the nose) is often used to help to confirm the diagnosis. Cultures and blood tests are usually necessary to confirm the disease.
Infections of ear, sinuses and eye
Primary infections of the external ear or sinuses, conjunctiva, eye, lids and intraocular orbits have been described. These types of generalized and disseminated cases of aspergillosis are rare. This condition is usually clinically diagnosed in a person with low immune defences {697}.
On the other hand, non-pulmonary colonization may occur in less debilitated patients, as eye Infections {1246; 1247}, external ear colonisation/infection {4319}. A. fumigatus is one of the most prevalent agents of fungal sinusitis {820; 176}. Sinusitis colonisation may lead to an allergic response {273}, to fungus balls or become invasive; complications may occur and be fatal {390}.
Virulence factors
This species, which plays an essential role in the ecosystem, is continuously inhaled by humans and is eliminated efficiently by natural intact barriers and adequate immune system. However, among immunocompromised hosts, A. fumigatus is the most common and life-threatening aerial fungal opportunistic pathogen {675}.
Aside from taking advantage of weakened host defences, A. fumigatus has specific biochemical and biological characteristics that confer to this fungus some enhanced opportunistic properties: the main virulence factors are its capacity to grow at 37°C and its toxins production.
More details
Since A. fumigatus can tolerate very high temperature, at the upper limit for eukaryotic organisms, this is believed to confer unique virulence properties to the organism and adaptation to the host’s parameters {673}.
A. fumigatus strains isolated from patients and environment produce metabolites considered as putative virulence factors: these include production of pigments, adhesion molecules present on the cell surface and secretion of hydrolytic enzymes and toxins {791; 4138; 4143; 4166; 4189}.
Mycotoxins present in clinical strains may contribute to the pathogenicity of A. fumigatus {299; 4146}, as they facilitate penetration of the fungus in the host’s cells. Other toxin related mechanisms have been studied, most relating to the impediment of the phagocytosis of the mould by the host’s cells {673; 794; 299; 675}. Pathogenicity of A. fumigatus could be facilitated by its toxic effects on macrophages {4104; 4137; 794; 4193} , neutrophils {4108} and leucocytes {4136}. Some strains of Aspergillus fumigatus inhibits phagocytosis by murine alveolar macrophages {4104; 4137}.
Gliotoxin is probably the primary toxin involved in these mechanisms. It has been suggested that this toxin may play an important role in the pathogenesis of aspergillosis as gliotoxin has immunosuppressive activity both in vitro and in vivo {791}. Gliotoxin from Aspergillus fumigatus seems to inhibit phagocytosis and the consequent killing of conidia by human neutrophils favouring the dissemination of A. fumigatus {4108}.
Specific settings
Nosocomial infections
Hundreds of single cases and outbreak cases of nosocomial infections due to A. fumigatus have been reported. Even small concentrations of spores have been associated with outbreaks, mainly due to A. fumigatus or A. flavus. Consequently, patients at risk should not be exposed to Aspergillus; environmental and infection control measures must be in place to limit the risk to patients {434; 1255}.
Infection control measures include cleaning, disinfection and filtration processes, as well as surveillance protocols in high risk situations {492; 4159}. Appropriate portable filtration units have proven to be useful in certain settings, even with short treatment duration {4103; 622}.
Virtually all outbreaks of nosocomial aspergillosis are attributed to airborne sources, mostly associated with construction work {434; 2465}. In order to limit this risk, construction work in a hospital setting should be done with strict protective measures {691; 434; 1255; 4265} because A. fumigatus has been isolated from the hospital environment in various places. Contaminated water systems, ventilation conduits and filtration systems as well as dust generated by construction work have been identified as contaminated with A. fumigatus {4100; 4153}.
The growing incidence of nosocomial invasive aspergillosis is also related with the development of new therapies and the presence of Aspergillus spores in the patients' vicinity. Construction, demolition or renovation and excavation works adjacent to or inside the hospital may be involved. Other environmental exposures such as contaminated ventilation systems and plumbing as well as less frequent sources such as potted plants in the patients’ room have been identified as significant source of Aspergillus spores {4156; 435; 434}.
Few cases of infection are iatrogenic in nature; catheters, injection sites and dialysis areas {4119; 4313}. Epidemiological investigations coupled to strain typing have also linked single cases and outbreaks to environmental sources.
More details
Construction works does influence Aspergillus infection rates; however, protective measures still needs to be studied {691}.
Aspergillosis in immunocompromised paediatric patients has been associated with building hygiene, design, and indoor air contamination {2074}. In one event, aspergillosis in immunocompromised paediatric patients may have been attributed to a defective disposal conduit door as well as the dispersal of a contaminated aerosol from the ward vacuum cleaner which had the highest measured concentrations of Aspergillus fumigatus in or around the building {2074}.
A hospital aerobiological survey found that the prevailing species was Aspergillus fumigatus which constituted on average 77.0% of total fungal strains isolated from the air of the hospital ward with the very distinct peak in November and the lowest value in May {487}.
High concentrations of airborne Aspergillus propagules were found in bathrooms, compared to that of the halls and rooms. Were water use was highest counts were higher (2.95 cfu/m³) as compared to patient rooms (0.78 cfu/m³; P=.05) and in hallways (0.61 cfu/m³) (p=.03) {351}.
A temporal survey of air contaminants showed that some strains can persist in the same environment for at least 6 months. In that study, patients with invasive aspergillosis were infected by a single strain over a 2 year period {56}.
Different strain typing methods have proven to be effective methods in outbreak investigations {4145} .The many outbreak studies have been able to link the cases either to a single common exposure or to multiple sources in the same time period; in some instances the investigators were only able to eliminate sources as being a common cause without being able to identify the source(s) {4128; 2439; 382; 4150; 385; 4186}.
The extrinsic and multiple causes of hospital contamination may explain the numerous problems the epidemiological investigations experience in linking clinical strains to fungal sources by different typing techniques {351; 384; 56; 4156}.
Occupational diseases
Respiratory problems have been associated with exposure to A. fumigatus in the workplace: allergic reactions, asthma and hypersensitivity pneumonitis (HP); reactions to toxins have also been reported. Most cases are seen in workers exposed to plant material or wastewater; they can occur as single cases or clusters following various work related exposures.
More details
The workers most at risk are:
- Farmers and silo workers {4093; 1625; 4180; 4179}
- Tobacco and herbs handlers {4125; 3266; 2603}
- Garbage collectors and recycling sorters {226; 4154}
- Landfill workers {1149; 1148; 3957}
- Workers in sewers and water treatment facilities {4113; 4112}
- Biofuel and compost plants {4102; 1151; 783; 1150; 4149}
- Workers exposed to wood dust and wood chips in sawmills and furniture factories {4099; 776; 4177}
- Plasterers exposed to esparto grass {208; 246; 1687}
- Office workers in contaminated buildings {4139}
Farmers and silo workers
Farmer’s lung disease is a very well known allergic disease, an hypersensitivity pneumonitis; it is caused by breathing in the dust from mouldy hay or any other contaminated crop (straw, corn, silage, grain, tobacco potatoes , herbs).
Farmers with cellular reactivity to airborne microbes suffer more frequently from work-related skin symptoms and allergic dermatitis. In one study, 75 farming students (49 males and 26 females aged 16-23 years) underwent dermatological, laryngological and pulmonary examination, skin prick tests with common and farm allergens and total IgE measurement. Tests with storage mites and fungal allergens also helped identify the cause of the symptoms {4180; 4179}.
Farmers and silo workers are also exposed to the mycotoxins contained in the grain and hay dust. These toxins are more abundant in stored products that self ferment.
Researchers have investigated the exposure of livestock and farm workers to mycotoxins during the last months of silage use, the mycoflora and the mycotoxins in a mature silage (11-months-old) {1625}. The screening of potentially toxigenic fungi isolated from the mature silage showed that six Fusarium species and Aspergillus fumigatus were able to produce mycotoxins on nutrient agar. Seven major mycotoxins were also searched in the corn silage. Among the three mycotoxins detected in the silage, gliotoxin, a strongly immunosuppressive mycotoxin, occurred in the mature silage at level up to 877 ppb, which was associated with the presence of A. fumigatus.
Tobacco and herbs handlers
Workers handling tobacco, potatoes and herbs can be exposed to mouldy plant material. In one study, people exposed to dust from herbs showed a higher frequency of positive skin reactions to microbial antigens as compared to the reference group {4125}. Precipitin tests also revealed greater reactivity to the environmental microbial antigens as compared to the reference group. Positive result rates of the Aspergillus fumigatus test were high (13.3%), as compared to the reference group having no positive result for any antigen. The risk of sensitization seems to be greatest among thyme farmers, who showed the highest positive response.
Garbage collectors and recycling sorters
A growing number of persons engaged in garbage collection and recycling separation might become endangered by high loads of bacteria and fungi {226}. In one case, the diagnosis was allergic bronchopulmonary aspergillosis (ABPA) including asthmatic responses as well as hypersensitivity pneumonitis (extrinsic allergic alveolitis) due to exposure to mouldy household waste. IgE- and IgG-antibodies were strongly positive for Aspergillus fumigatus; antibodies persisted at least 2 years. A. fumigatus extract containing gliotoxin resulted in an immediate-type asthmatic reaction.
Field studies of microbial exposure associated with the collection of residential garbage have shown that during warm periods in the summertime, the concentration of A. fumigatus increased, reaching up to 90,000 cfu/m³ {4154}.
Landfill workers are exposed to the same bioaerosols as garbage collectors and recycling sorters. One study has suggested that A. fumigatus strains developed in stored wastes produce strong cellulotytic enzymes which need further studies for their potential allergenic and/or immunotoxic effects of these proteins on exposed workers {3957}.
Workers in sewers and water treatment facilities
Pathogenic A. fumigatus can be found at all stages of wastewater treatment {4112}. Sewage sludge is the main source of airborne emission at workplaces. It is suggested that personal protective equipment should be used by workers. Exposure assessment to bioaerosols among sewer workers showed the presence of A. fumigatus which is classified as a harmful biological agent (occupational risk group 2) {4113}.
There are species-specific profiles of mycotoxins produced in cultures and associated with airborne fungi derived from biowaste {1148}: A. fumigatus strains, as well as other fungi, have the potential to produce mycotoxins and non-volatile secondary metabolites. Some of the mycotoxins (fumigaclavine C, tryptoquivaline, and trypacidin), characteristic for A. fumigatus, were found in conidial extracts, but highly toxic compounds such as gliotoxin and fumitremorgens were not present.
Biofuel and compost plants, peat moss
A. fumigatus is one of the most frequent species in the air of compost plants and biological waste treatment plants {4102}. A wide range of microbial volatile organic compounds (MVOC) and mycotoxins, were found in pure cultures of freshly isolated strains of A. fumigatus. Tryptoquivaline, a compound with tremorgenic properties, and trypacidin, for which no toxic properties are described, were found in native bioaerosols in a compost facility {783}. People working at least 30 minutes a day with woodchips were exposed to a median value of A. fumigatus of 6.7x104 cfu m³ and a median value of 70x104 spores m³. Consequently, this working environment may cause respiratory disorders {4149}. A. fumigatus was also associated with peat moss in nature as well as in its later usage. Respiratory exposure to organic dust generated by peat moss bedding may also induce chronic pulmonary diseases both in farmers and horses {4149} .
Workers exposed to wood dust and wood chips in sawmills and furniture factories.
Transformation of wood and wood products generates organic dust containing moulds. Allergenic fungi (mostly Aspergillus fumigatus and Penicillium spp.) are found in sawmills and debarking processes {776}; they are prevailing microorganisms in the air of the fiberboard factories {858}. Early allergic reactions to microorganisms, mostly A. fumigates, associated with wood dust, are common among workers of furniture industry {4099}.
Plasterers exposed to esparto grass
Espartosis is a type of hypersensitivity pneumonitis which frequently affects construction workers handling esparto fibres used as support material inside gypsum plaster {208; 246; 1687; 200}. A. fumigatus is recognized as a causal agent in hypersensitivity pneumonitis due to esparto grass exposure; however, other antigenic sources such as other fungi and esparto grass fibres, also appear to play a role in the genesis of this disease {208}.
Diagnostic tools
Cultures
Direct examination of sinus secretions showing typical septate hyphal fragments is indicative of a fungal sinusitis but must be followed by the proper cultures to confirm A. fumigatus as the aetiological agent.
The diagnosis of deep wound infections can be confirmed by definite microbiological diagnosis i.e., presence of A. fumigatus on cultures of deep operative wound samples.
As for suspected allergic bronchopulmonary aspergillosis (ABPA) cases, cultures may be negative; when positive they are not sufficient to confirm the diagnosis which should be based on the characteristic clinical and laboratory findings of ABPA; specific hypersensitivity can be verified by immunodiagnostic tests (see below).
Histopathology
Methanamine silver stain is most helpful in obtaining good contrast between fungal structures and background tissue. In pulmonary biopsies, the characteristic sporulating Aspergillus conidiophores may be occasionally seen in surface tissue from lung cavities or other lesions containing airspace. Other sectioned material will show septate hyphal forms only (broad hyphae of 3-5 µ), and may show branching clusters of radiate outward hyphal strands. In cases of eye infections, sections show giant cells containing septate hyphae accompanied by polymorphonuclear cells and macrophages.
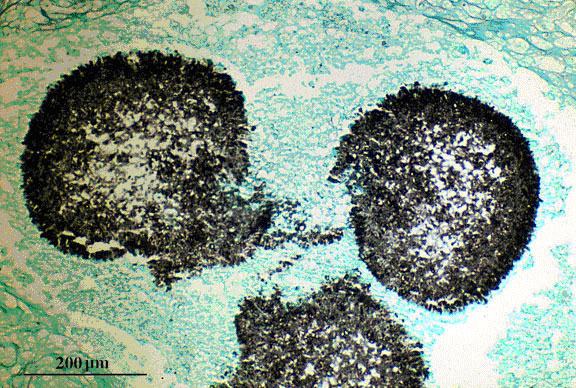
Fig 1. Grocott’s methenamine silver (Giemsa - GMS) stained tissue section of lung showing fungal balls of hyphae of Aspergillus fumigates (aspergilloma).
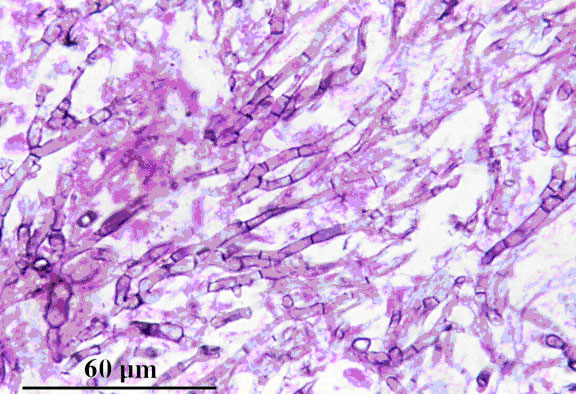
Fig. 2. Grocott’s methenamine silver (GMS) stained tissue section of lung showing dichotomously branched, septate hyphae of Aspergillus fumigatus.
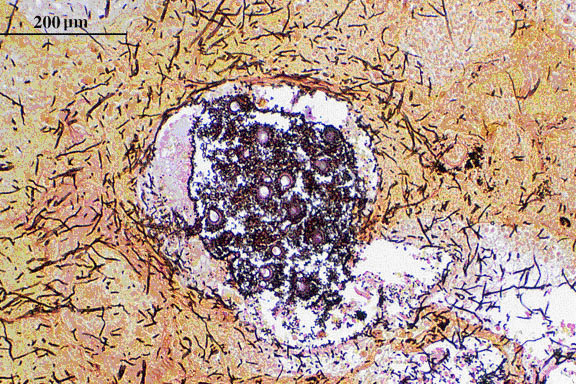
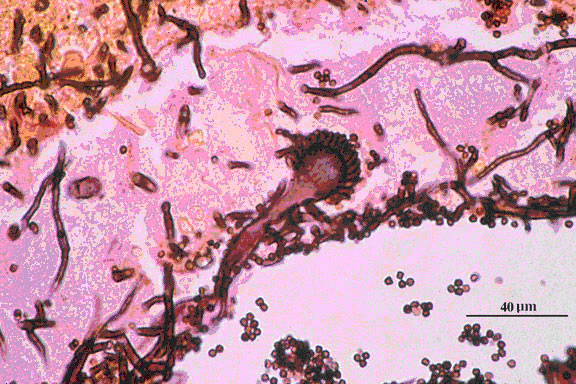
Fig 3. Grocott’s methenamine silver (Giemsa - GMS) stained tissue sections showing Aspergillus fumigatus in lung tissue; note conidial heads forming in an alveolus.
Immunodiagnosis
Blood tests are available to complement the diagnosis of an infection, hypersensitivity pneumonitis or allergic reaction. Most antibody tests lack sensitivity {251}. The latex antigen test is highly specific but also lacks sensitivity. In a study designed to evaluate health effect to mould exposure after moisture damage {199}, an association between episodes of sinusitis during the previous 12 months and a significantly higher mould-specific IgG to 10 moulds, including A. Fumigates was reported.
Ouchterlony IgG precipitin are specific but not as sensitive as the newer RAST tests. Skin tests are readily available to diagnose A. fumigatus allergy. Results may vary depending on the type of extract used to perform the test. Antigenic extracts of Aspergillus fumigatus are available for the IgE-RAST and the double immunodiffusion tests, as single extracts or pooled antigens.
| Test | IgE | IgG | Antigens | Other |
|---|---|---|---|---|
| Skin Tests | X | X | ||
| RAST-IgE | X | |||
| RAST-IgG | X | |||
| ELISA-ELIFA | Experimental | |||
| Immunodiffusion | Experimental | |||
| Immunofluorescence | Experimental | |||
| Complement fixation | Experimental | |||
| PCR | ||||
| Other |
More details
Aspergillus fumigatus allergens are part of the American Food and Drug Administration (FDA) surveillance program and part of the Biological Product Deviation Reports mould list {3285}:
- GJ08 - Aspergillus amsterdami
- GJ09 - Aspergillus clavatus
- GJ10 - Aspergillus flavipes
- GJ11 - Aspergillus flavus
- GJ12 - Aspergillus fumigatus
- GJ13 - Aspergillus glaucus
- GJ14 - Aspergillus nidulans
- GJ15 - Aspergillus niger
- GJ16 - Aspergillus ochraceus
- GJ17 - Aspergillus restrictus
- GJ18 - Aspergillus sydowi
- GJ19 - Aspergillus terreus
- GJ20 - Aspergillus ustus
- GJ21 - Aspergillus versicolor
Bibliography
- 54. Morhart, M., Rennie, R., Ziola, B., Bow, E., and Louie, T. J. (1994). Evaluation of enzyme immunoassay for Candida cytoplasmic antigens in neutropenic cancer patients. J Clin Microbiol. 32[3], 766-776.
- 56. Girardin, H., Sarfati, J., Traore, F., Dupouy, Camet J., Derouin, F., and Latge, J. P. (1994). Molecular epidemiology of nosocomial invasive aspergillosis. J Clin Microbiol. 32[3], 684-690.
- 63. Kern, M E. (1985). Medical mycology a self-instructional text. -239 p. Philadelphia, F. A. Davis Company.
- 142. Bornehag, C. G., Blomquist, G., Gyntelberg, F., Jarvholm, B., Malmberg, P., Nordvall, L., Nielsen, A., Pershagen, G., and Sundell, J. (2001). Dampness in buildings and health. Nordic interdisciplinary review of the scientific evidence on associations between exposure to "dampness" in buildings and health effects (NORDDAMP). Indoor.Air. 11[2], 72-86.
- 154. Verhoeff, A. P. and Burge, H. A. (1997). Health risk assessment of fungi in home environments. Ann Allergy Asthma Immunol. 78[6], 544-554.
- 165. Dales, R. E., Burnett, R., and Zwanenburg, H. (1991). Adverse health effects among adults exposed to home dampness and molds. Am Rev Respir.Dis. 143[3], 505-509.
- 174. Kurup, V. P., Knutsen, A. P., Moss, R. B., and Bansal, N. K. (2006). Specific antibodies to recombinant allergens of Aspergillus fumigatus in cystic fibrosis patients with ABPA. Clin Mol.Allergy. 4:11., 11.
- 176. Shah, A. and Panjabi, C. (2006). Contemporaneous occurrence of allergic bronchopulmonary aspergillosis, allergic Aspergillus sinusitis, and aspergilloma. Ann Allergy Asthma Immunol. 96[6], 874-878.
- 186. Kurup, V. P. (2005). Aspergillus antigens: which are important? Med Mycol. 43 Suppl 1:S189-96., S189-S196.
- 189. Gamboa, P. M., Urbaneja, F., Olaizola, I., Boyra, J. A., Gonzalez, G., Antepara, I., Urrutia, I., Jauregui, I., and Sanz, M. L. (2005). Specific IgG to Thermoactynomices vulgaris, Micropolyspora faeni and Aspergillus fumigatus in building workers exposed to esparto grass (plasterers) and in patients with esparto-induced hypersensitivity pneumonitis. J Investig.Allergol.Clin Immunol. 15[1]
- 199. Patovirta, R. L., Reiman, M., Husman, T., Haverinen, U., Toivola, M., and Nevalainen, A. (2003). Mould specific IgG antibodies connected with sinusitis in teachers of mould damaged school: a two-year follow-up study. Int J Occup.Med Environ Health. 16[3], 221-230.
- 200. Moreno-Ancillo, A., Dominguez-Noche, C., Carmen Gil-Adrados, A., and Cosmes, P. M. (2003). Familial presentation of occupational hypersensitivity pneumonitis caused by aspergillus-contaminated esparto dust. Allergol.Immunopathol.(Madr.). 31[5], 294-296.
- 206. Sarma, U. P., Kurup, V. P., and Madan, T. (2003). Immunodiagnosis of ABPA. Front Biosci. 8:s1187-98., s1187-s1198.
- 208. Cruz, M. J., Morell, F., Roger, A., Munoz, X., and Rodrigo, M. J. (2003). [Hypersensitivity pneumonitis in construction plasterers (espartosis): study of 20 patients]. Med Clin (Barc.). 120[15], 578-583.
- 226. Allmers, H., Huber, H., and Baur, X. (2000). Two year follow-up of a garbage collector with allergic bronchopulmonary aspergillosis (ABPA). Am J Ind.Med. 37[4], 438-442.
- 227. Erkinjuntti-Pekkanen, R., Reiman, M., Kokkarinen, J. I., Tukiainen, H. O., and Terho, E. O. (1999). IgG antibodies, chronic bronchitis, and pulmonary function values in farmer's lung patients and matched controls. Allergy. 54[11], 1181-1187.
- 228. Eppinger, T. M., Greenberger, P. A., White, D. A., Brown, A. E., and Cunningham-Rundles, C. (1999). Sensitization to Aspergillus species in the congenital neutrophil disorders chronic granulomatous disease and hyper-IgE syndrome. J Allergy Clin Immunol. 104[6], 1265-1272.
- 230. Da Broi, U., Orefice, U., Cahalin, C., Bonfreschi, V., and Cason, L. (1999). ARDS after double extrinsic exposure hypersensitivity pneumonitis. Intensive Care Med. 25[7], 755-757.
- 231. Muller-Wening, D., Renck, T., and Neuhauss, M. (1999). [Wood chip alveolitis]. Pneumologie. 53[7], 364-368.
- 233. Skov, M., Pressler, T., Jensen, H. E., Hoiby, N., and Koch, C. (1999). Specific IgG subclass antibody pattern to Aspergillus fumigatus in patients with cystic fibrosis with allergic bronchopulmonary aspergillosis (ABPA). Thorax. 54[1], 44-50.
- 246. Hinojosa, M., Fraj, J., De la, Hoz B., Alcazar, R., and Sueiro, A. (1996). Hypersensitivity pneumonitis in workers exposed to esparto grass (Stipa tenacissima) fibers. J Allergy Clin Immunol. 98[5 Pt 1], 985-991.
- 251. Kappe, R., Schulze-Berge, A., and Sonntag, H. G. (1996). Evaluation of eight antibody tests and one antigen test for the diagnosis of invasive aspergillosis. Mycoses. 39[1-2], 13-23.
- 258. Collins, L. A., Samore, M. H., Roberts, M. S., Luzzati, R., Jenkins, R. L., Lewis, W. D., and Karchmer, A. W. (1994). Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 170[3], 644-652.
- 260. Wang, J. M., Denis, M., Fournier, M., and Laviolette, M. (1994). Experimental allergic bronchopulmonary aspergillosis in the mouse: immunological and histological features. Scand J Immunol. 39[1], 19-26.
- 265. Eduard, W., Sandven, P., and Levy, F. (1993). Serum IgG antibodies to mold spores in two Norwegian sawmill populations: relationship to respiratory and other work-related symptoms. Am J Ind.Med. 24[2], 207-222.
- 271. Ojanen, T. (1992). Class specific antibodies in serodiagnosis of farmer's lung. Br J Ind.Med. 49[5], 332-336.
- 273. Corey, J. P. (1992). Allergic fungal sinusitis. Otolaryngol.Clin North Am. 25[1], 225-230.
- 275. Banerjee, B., Joshi, A. P., Sarma, P. U., and Roy, S. (1992). Evaluation of clinico-immunological parameters in pediatric ABPA patients. Indian J Pediatr. 59[1], 109-114.
- 293. (2006). Health concerns associated with mold in water-damaged homes after Hurricanes Katrina and Rita--New Orleans area, Louisiana, October 2005. MMWR Morb.Mortal.Wkly.Rep. %20;55[2], 41-44.
- 299. Kosalec, I. and Pepeljnjak, S. (2005). Mycotoxigenicity of clinical and environmental Aspergillus fumigatus and A. flavus isolates. Acta Pharm. 55[4], 365-375.
- 300. Kosalec, I., Klaric, M. S., and Pepeljnjak, S. (2005). Verruculogen production in airborne and clinical isolates of Aspergillus fumigatus Fres. Acta Pharm. 55[4], 357-364.
- 338. Dimopoulos, G., Piagnerelli, M., Berre, J., Eddafali, B., Salmon, I., and Vincent, J. L. (2003). Disseminated aspergillosis in intensive care unit patients: an autopsy study. J Chemother. 15[1], 71-75.
- 351. Anaissie, E. J., Stratton, S. L., Dignani, M. C., Summerbell, R. C., Rex, J. H., Monson, T. P., Spencer, T., Kasai, M., Francesconi, A., and Walsh, T. J. (2002). Pathogenic Aspergillus species recovered from a hospital water system: a 3-year prospective study. Clin Infect Dis. 34[6], 780-789.
- 354. Grow, W. B., Moreb, J. S., Roque, D., Manion, K., Leather, H., Reddy, V., Khan, S. A., Finiewicz, K. J., Nguyen, H., Clancy, C. J., Mehta, P. S., and Wingard, J. R. (2002). Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 29[1], 15-19.
- 355. Smedbold, H. T., Ahlen, C., Norback, D., and Hilt, B. (2001). Sign of eye irritation in female hospital workers and the indoor environment. Indoor.Air. 11[4], 223-231.
- 363. Oren, I., Haddad, N., Finkelstein, R., and Rowe, J. M. (2001). Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol. 66[4], 257-262.
- 372. Kontoyiannis, D. P., Wessel, V. C., Bodey, G. P., and Rolston, K. V. (2000). Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 30[6], 851-856.
- 376. Skov, M., Koch, C., Reimert, C. M., and Poulsen, L. K. (2000). Diagnosis of allergic bronchopulmonary aspergillosis (ABPA) in cystic fibrosis. Allergy. 55[1], 50-58.
- 382. Leenders, A. C., van Belkum, A., Behrendt, M., Luijendijk, A., and Verbrugh, H. A. (1999). Density and molecular epidemiology of Aspergillus in air and relationship to outbreaks of Aspergillus infection. J Clin Microbiol. 37[6], 1752-1757.
- 384. Chazalet, V., Debeaupuis, J. P., Sarfati, J., Lortholary, J., Ribaud, P., Shah, P., Cornet, M., Vu, Thien H., Gluckman, E., Brucker, G., and Latge, J. P. (1998). Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J Clin Microbiol. 36[6], 1494-1500.
- 385. Radford, S. A., Johnson, E. M., Leeming, J. P., Millar, M. R., Cornish, J. M., Foot, A. B., and Warnock, D. W. (1998). Molecular epidemiological study of Aspergillus fumigatus in a bone marrow transplantation unit by PCR amplification of ribosomal intergenic spacer sequences. J Clin Microbiol. 36[5], 1294-1299.
- 390. Nenoff, P., Horn, L. C., Schwenke, H., Mierzwa, M., Rieske, K., and Haustein, U. F. (1996). [Invasive mold infections in the university clinics of Leipzig in the period from 1992-1994]. Mycoses. 39 Suppl 1:107-12., 107-112.
- 406. Conant, N F, Smith, D T, Baker, R D, and Callaway, J L. (1971). Manual of clinical mycology. 3th edition, -755 p. Philadelphia-London-Toronto, W. B. Saunders Company.
- 413. Storey, E, Dangman, K H, Schenck, P, DeBernardo, R L, Yang, C S, Bracker, A, and Hodgson, M J. (2004). Guidance for clinicians on the recognition and management of health effects related to mold exposure and moisture indoors. -58 p. Farmington, Center for Indoor Environment and Health, University of Connecticut Health Center.
- 415. St-Germain, G and Summerbell, R. (1996). Champignons filamenteux d'intérêt médical. Caractéristiques et idenfication. -314 p. Belmont, Star Publishing Company.
- 434. Santé Canada. (2001). Infections nosocomiales chez les patients d'établissements de santé liées aux travaux de constuction. Atténuer le risque d'aspergillose, de légionellose et d'autres infections. Relevé des maladies transmissibles du Canada 27S2, -46 p. Ministère de la San
- 435. Santé Canada. (1999). Infections nosocomiales liées aux travaux de construction : atténuer le risque d'aspergillose, de légionellose et d'autres infections chez des patients hospitalisés. 6e version, -56 p.
- 487. Augustowska, M. and Dutkiewicz, J. (2006). Variability of airborne microflora in a hospital ward within a period of one year. Ann Agric.Environ Med. 13[1], 99-106.
- 491. Hensley, M. E., Ke, W., Hayden, R. T., Handgretinger, R., and McCullers, J. A. (2004). Levels of total fungus and Aspergillus on a pediatric hematopoietic stem cell transplant unit. J Pediatr.Oncol Nurs. 21[2], 67-78.
- 492. Gangneux, J. P., Poirot, J. L., Morin, O., Derouin, F., Bretagne, S., Datry, A., Kauffmann-Lacroix, C., Paugam, A., Chandenier, J., Bouakline, A., Bordes, M., Chachaty, E., Dupeyron, C., Grawey, I., Lecso, G., Lortholary, J., Mourlhou, P., Nesa, D., Saheb (2002). [Mycologic surveillance of the environment for preventive invasive aspergillosis. Proposals for standardization of the methodologies and implementation]. Presse Med. 31[18], 841-848.
- 512. Newman, M. J. (2002). Neonatal intensive care unit: reservoirs of nosocomial pathogens. West Afr.J Med. 21[4], 310-312.
- 517. Alberti, C., Bouakline, A., Ribaud, P., Lacroix, C., Rousselot, P., Leblanc, T., and Derouin, F. (2001). Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J Hosp.Infect. 48[3], 198-206.
- 560. Singh, B. P., Banerjee, B., and Kurup, V. P. (2003). Aspergillus antigens associated with allergic bronchopulmonary aspergillosis. Front Biosci. 8:s102-9., s102-s109.
- 566. Norback, D., Walinder, R., Wieslander, G., Smedje, G., Erwall, C., and Venge, P. (2000). Indoor air pollutants in schools: nasal patency and biomarkers in nasal lavage. Allergy. 55[2], 163-170.
- 594. Claeson, A. S., Levin, J. O., Blomquist, G., and Sunesson, A. L. (2002). Volatile metabolites from microorganisms grown on humid building materials and synthetic media. J Environ Monit. 4[5], 667-672.
- 595. Nieminen, S. M., Karki, R., Auriola, S., Toivola, M., Laatsch, H., Laatikainen, R., Hyvarinen, A., and Von Wright, A. (2002). Isolation and identification of Aspergillus fumigatus mycotoxins on growth medium and some building materials. Appl.Environ Microbiol. 68[10], 4871-4875.
- 598. Gao, P., Korley, F., Martin, J., and Chen, B. T. (2002). Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. AIHA.J (Fairfax., Va.). 63[2], 135-140.
- 605. Gravesen, S., Nielsen, P. A., Iversen, R., and Nielsen, K. F. (1999). Microfungal contamination of damp buildings--examples of risk constructions and risk materials. Environ Health Perspect. 107 Suppl 3:505-8., 505-508.
- 622. Sixt, N., Dalle, F., Lafon, I., Aho, S., Couillault, G., Valot, S., Calinon, C., Danaire, V., Vagner, O., Cuisenier, B., Sautour, M., Besancenot, J. P., L'ollivier, C., Caillot, D., and Bonnin, A. (2006). Reduced fungal contamination of the indoor environment with the Plasmairtrade mark system (Airinspace). J Hosp.Infect. .
- 670. Godish, T. J. and Godish, D. R. (2006). Mold infestation of wet spray-applied cellulose insulation. J Air Waste Manag.Assoc. 56[1], 90-95.
- 673. Bhabhra, R. and Askew, D. S. (2005). Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med Mycol. 43 Suppl 1:S87-93., S87-S93.
- 675. Latge, J. P. (2003). Aspergillus fumigatis, a saprotrophic pathogenic fungus. Mycologist. 17[Part 2], 56.
- 683. Cornillet, A., Camus, C., Nimubona, S., Gandemer, V., Tattevin, P., Belleguic, C., Chevrier, S., Meunier, C., Lebert, C., Aupee, M., Caulet-Maugendre, S., Faucheux, M., Lelong, B., Leray, E., Guiguen, C., and Gangneux, J. P. (2006). Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 43[5], 577-584.
- 684. Singh, N., Limaye, A. P., Forrest, G., Safdar, N., Munoz, P., Pursell, K., Houston, S., Rosso, F., Montoya, J. G., Patton, P. R., Del Busto, R., Aguado, J. M., Wagener, M. M., and Husain, S. (2006). Late-onset invasive aspergillosis in organ transplant recipients in the current era. Med Mycol. 44[5], 445-449.
- 685. Bhatti, Z., Shaukat, A., Almyroudis, N. G., and Segal, B. H. (2006). Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia. 162[1], 1-15.
- 691. Cooper, E. E., O'Reilly, M. A., Guest, D. I., and Dharmage, S. C. (2003). Influence of building construction work on Aspergillus infection in a hospital setting. Infect Control Hosp.Epidemiol. 24[7], 472-476.
- 697. Tabbara, K. F. and al Jabarti, A. L. (1998). Hospital construction-associated outbreak of ocular aspergillosis after cataract surgery. Ophthalmology. 105[3], 522-526.
- 711. Anyanwu, E., Campbell, A. W., and High, W. (2002). Brainstem auditory evoked response in adolescents with acoustic mycotic neuroma due to environmental exposure to toxic molds. Int J Adolesc Med Health. 14[1], 67-76.
- 724. Samson, RA, Hoekstra, ES, and et al. (1984). Introduction to food and airbone fungi. 6th, -389 p. Baarn, Centralalbureau voor Schimmellcultures, Institute of the Royal Netherlands Academy of Arts and Sciences.
- 765. Panaccione, D. G. and Coyle, C. M. (2005). Abundant respirable ergot alkaloids from the common airborne fungus Aspergillus fumigatus. Appl.Environ Microbiol. 71[6], 3106-3111.
- 766. Park, J. H., Cox-Ganser, J., Rao, C., and Kreiss, K. (2006). Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 16[3], 192-203.
- 776. Dutkiewicz, J., Krysinska-Traczyk, E., Prazmo, Z., Skorska, C., and Sitkowska, J. (2001). Exposure to airborne microorganisms in Polish sawmills. Ann Agric Environ Med. 8[1], 71-80.
- 777. Land, C. J., Hult, K., Fuchs, R., Hagelberg, S., and Lundstrom, H. (1987). Tremorgenic mycotoxins from Aspergillus fumigatus as a possible occupational health problem in sawmills. Appl Environ Microbiol. 53[4], 787-790.
- 779. Land, C. J., Lundstrom, H., and Werner, S. (1993). Production of tremorgenic mycotoxins by isolates of Aspergillus fumigatus from sawmills in Sweden. Mycopathologia. 124[2], 87-93.
- 783. Fischer, G., Muller, T., Ostrowski, R., and Dott, W. (1999). Mycotoxins of Aspergillus fumigatus in pure culture and in native bioaerosols from compost facilities. Chemosphere. 38[8], 1745-1755.
- 791. Belkacemi, L., Barton, R. C., Hopwood, V., and Evans, E. G. (1999). Determination of optimum growth conditions for gliotoxin production by Aspergillus fumigatus and development of a novel method for gliotoxin detection. Med Mycol. 37[4], 227-233.
- 794. Kamei, K. and Watanabe, A. (2005). Aspergillus mycotoxins and their effect on the host. Med Mycol. 43 Suppl 1:S95-9., S95-S99.
- 812. Fungal Research Trust. (2007). The Aspergillus Web-Site. http://www.aspergillus.org.uk . 2007.
- 820. Dolen, W. K. (2006). Risk factors for allergic Aspergillus sinusitis. Med Mycol. 44 Suppl:273-5., 273-275.
- 822. Almeida, M. B., Bussamra, M. H., and Rodrigues, J. C. (2006). ABPA diagnosis in cystic fibrosis patients: the clinical utility of IgE specific to recombinant Aspergillus fumigatus allergens. J Pediatr.(Rio J). 82[3], 215-220.
- 846. Jacobs, R. L., Andrews, C. P., and Coalson, J. J. (2005). Hypersensitivity pneumonitis: beyond classic occupational disease-changing concepts of diagnosis and management. Ann Allergy Asthma Immunol. 95[2], 115-128.
- 854. Lugauskas, A., Krikstaponis, A., and Sveistyte, L. (2004). Airborne fungi in industrial environments--potential agents of respiratory diseases. Ann Agric.Environ Med. 11[1], 19-25.
- 858. Dutkiewicz, J., Olenchock, S., Krysinska-Traczyk, E., Skorska, C., Sitkowska, J., and Prazmo, Z. (2001). Exposure to airborne microorganisms in fiberboard and chipboard factories. Ann Agric.Environ Med. 8[2], 191-199.
- 874. Santé Canada. (2007). Protéger la santé des populations vulnérables. Bulletin d'information 5[décembre 2006], 1-2.
- 931. Botterel, F., Cordonnier, C., Barbier, V., Wingerstmann, L., Liance, M., Coste, A., Escudier, E., and Bretagne, S. (2002). Aspergillus fumigatus causes in vitro electrophysiological and morphological modifications in human nasal epithelial cells. Histol.Histopathol. 17[4], 1095-1101.
- 946. Santé Canada. (2007). Ligne directrice sur la qualité de l'air intérieur résidentiel : moisissures. Santé Canada and Ministère de la santé. Gazette du Canada partie 1, 4330-4331. Gouvernenement du Canada. 2-10-0070.
- 979. Cormier, Y., Laviolette, M., Cantin, A., Tremblay, G. M., and Begin, R. (1993). Fibrogenic activities in bronchoalveolar lavage fluid of farmer's lung. Chest. 104[4], 1038-1042.
- 981. Cormier, Y., Fournier, M., and Laviolette, M. (1993). Farmer's fever. Systemic manifestation of farmer's lung without lung involvement. Chest. 103[2], 632-634.
- 989. Centre de recherche sur la conservation des documents graphiques. (2007). Moisissures et biens culturels. Ministère de la culture et de la Communication, France .
- 991. Pitt, J. I. (1989). Recent developments in the study of Penicillium and Aspergillus systematics. J.Appl.Bact.Symp. Suppi. 37S45S.
- 1052. Raper, K. B. and Fennell, D. I. (1965). The Genus Aspergillus. Baltimore, Md, The Williams & Wilkins Co.
- 1089. Bart-Delabesse E and Latgé, JP. (2003). Ecology and genetics diversity of Aspergillus fumigatus. Domer JE & Kobayashi GS (eds). In The Mycota. [XII. Human Fungal Pathogens]. Berlin, Heidelgerg, Springer-Verlag.
- 1090. Ascioglu, S., Rex, JH, de Pauw, B, Bennett, J. E., Bille, J., and Crokaert, F. (2002). Defining opportunistic invasive fungal infections in immunocompromised patients, with cancer and hematopoietic stem cell, transplants: an international consensus. Clin Infect Dis 34, 7-14.
- 1091. Roussel, S., Reboux, G., Dalphin, J. C., Pernet, D., Laplante, J. J., Millon, L., and Piarroux, R. (2005). Farmer's lung disease and microbiological composition of hay: a case-control study. Mycopathologia. 160[4], 273-279.
- 1100. Dangman, K. H., Cole, S. R., Hodgson, M. J., Kuhn, C., Metersky, M. L., Schenck, P., and Storey, E. (2002). The hypersensitivity pneumonitis diagnostic index: use of non-invasive testing to diagnose hypersensitivity pneumonitis in metalworkers. Am J Ind.Med. 42[2], 150-162.
- 1103. Reboux, G., Piarroux, R., Mauny, F., Madroszyk, A., Millon, L., Bardonnet, K., and Dalphin, J. C. (2001). Role of molds in farmer's lung disease in Eastern France. Am J Respir.Crit Care Med. 163[7], 1534-1539.
- 1108. Mackiewicz, B., Skorska, C., Dutkiewicz, J., Michnar, M., Milanowski, J., Prazmo, Z., Krysinska-Traczyk, E., and Cisak, E. (1999). Allergic alveolitis due to herb dust exposure. Ann Agric.Environ Med. 6[2], 167-170.
- 1148. Fischer, G., Muller, T., Schwalbe, R., Ostrowski, R., and Dott, W. (2000). Species-specific profiles of mycotoxins produced in cultures and associated with conidia of airborne fungi derived from biowaste. Int J Hyg.Environ Health. 203[2], 105-116.
- 1149. Fischer, G., Muller, T., Schwalbe, R., Ostrowski, R., and Dott, W. (2000). Exposure to airborne fungi, MVOC and mycotoxins in biowaste-handling facilities. Int J Hyg.Environ Health. 203[2], 97-104.
- 1150. Fischer, G., Muller, T., Ostrowski, R., Schwalbe, R., and Dott, W. (1999). [Mycotoxins as exposure parameters in bioaerosols of composting sites]. Schriftenr.Ver.Wasser Boden Lufthyg. 104:149-62., 149-162.
- 1151. Fischer, G., Schwalbe, R., Moller, M., Ostrowski, R., Hollender, J., and Dott, W. (1998). [Airborne molds and their metabolites at workplaces in composting plants]. Mycoses. 41 Suppl 1:51-5., 51-55.
- 1243. Rutqvist, L. and Persson, P. A. (1966). Studies on Aspergillus fumigatus, experimental mycotoxicosis in mice, chicks and pigs wth the appearance, in pigs, of perirenal edema. Acta Vet.Scand. 7[1], 21-34.
- 1246. Matsuo, T., Nakagawa, H., and Matsuo, N. (1995). Endogenous Aspergillus endophthalmitis associated with periodontitis. Ophthalmologica. 209[2], 109-111.
- 1247. Matsuo, T., Notohara, K., and Yamadori, I. (2005). Aspergillosis causing bilateral optic neuritis and later orbital apex syndrome. Jpn.J Ophthalmol. 49[5], 430-431.
- 1255. U.S.Department of Health and Human Services and Centers for Disease Control and Prevention. (2003). Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Comittee (HICPAC). -233 p. Atlanta, Centers for Disease Control and Prevention.
- 1287. Knutsen, A. P., Hutcheson, P. S., Slavin, R. G., and Kurup, V. P. (2004). IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 59[2], 198-203.
- 1399. Koivikko, A., Viander, M., and Lanner, A. (1991). Use of the extended Phadebas RAST panel in the diagnosis of mould allergy in asthmatic children. Allergy. 46[2], 85-91.
- 1420. Immonen, J., Meklin, T., Taskinen, T., Nevalainen, A., and Korppi, M. (2001). Skin-prick test findings in students from moisture- and mould-damaged schools: a 3-year follow-up study. Pediatr Allergy Immunol. 12[2], 87-94.
- 1625. Richard, E., Heutte, N., Sage, L., Pottier, D., Bouchart, V., Lebailly, P., and Garon, D. (2007). Toxigenic fungi and mycotoxins in mature corn silage. Food Chem Toxicol. 45[12], 2420-2425.
- 1687. Moreno-Ancillo, A., Padial, M. A., Lopez-Serrano, M. C., and Granado, S. (1997). Hypersensitivity pneumonitis due to inhalation of fungi-contaminated esparto dust in a plaster worker. Allergy Asthma Proc. 18[6], 355-357.
- 1982. Aleksandrowicz, J. and Smyk, B. (1973). The association of neoplastic diseases and mycotoxins in the environment. Tex.Rep Biol Med. 31[4], 715-726.
- 2019. Malling, H. J., Agrell, B., Croner, S., Dreborg, S., Foucard, T., Kjellman, M., Koivikko, A., Roth, A., and Weeke, B. (1985). Diagnosis and immunotherapy of mould allergy. I. Screening for mould allergy. Allergy. 40[2], 108-114.
- 2074. Anderson, K., Morris, G., Kennedy, H., Croall, J., Michie, J., Richardson, M. D., and Gibson, B. (1996). Aspergillosis in immunocompromised paediatric patients: associations with building hygiene, design, and indoor air. Thorax. 51[3], 256-261.
- 2111. Mori, H., Abe, F., Furukawa, S., Furukawa, S., Sakai, F., Hino, M., and Fujii, T. (2003). FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC) II. Biological activities in animal models. J Antibiot.(Tokyo). 56[2], 80-86.
- 2123. Walsh, T. J. and Groll, A. H. (1999). Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl.Infect Dis. 1[4], 247-261.
- 2245. Storms, W. W. (1978). Occupational hypersensitivity lung disease. J Occup Med. 20[12], 823-824.
- 2439. Leenders, A., van, Belkum A., Janssen, S., de, Marie S., Kluytmans, J., Wielenga, J., Lowenberg, B., and Verbrugh, H. (1996). Molecular epidemiology of apparent outbreak of invasive aspergillosis in a hematology ward. J Clin Microbiol. 34[2], 345-351.
- 2465. Vonberg, R. P. and Gastmeier, P. (2006). Nosocomial aspergillosis in outbreak settings. J Hosp Infect. 63[3], 246-254.
- 2481. Anke, H., Kolthoum, I., and Laatsch, H. (1980). Metabolic products of microorganisms. 192. The anthraquinones of the Aspergillus glaucus group. II. Biological activity. Arch Microbiol. 126[3], 231-236.
- 2553. Hueso, Gutierrez P., Jimenez, Alvarez S., Gil-Carcedo, Sanudo E., Gil-Carcedo Garcia, L. M., Ramos, Sanchez C., and Vallejo Valdezate, L. A. (2005). [Presumption diagnosis: otomycosis. A 451 patients study]. Acta Otorrinolaringol.Esp. 56[5], 181-186.
- 2603. Skorska, C., Sitkowska, J., Krysinska-Traczyk, E., Cholewa, G., and Dutkiewicz, J. (2005). Exposure to airborne microorganisms, dust and endotoxin during processing of valerian roots on farms. Ann Agric Environ Med. 12[1], 119-126.
- 2680. Skorska, C., Sitkowska, J., Krysinska-Traczyk, E., Cholewa, G., and Dutkiewicz, J. (2005). Exposure to airborne microorganisms, dust and endotoxin during processing of peppermint and chamomile herbs on farms. Ann Agric Environ Med. 12[2], 281-288.
- 2740. Aira, M. J., Rodriguez-Rajo, F., and Jato, V. (2008). 47 annual records of allergenic fungi spore: predictive models from the NW Iberian Peninsula. Ann Agric Environ Med. 15[1], 91-98.
- 2943. Oh, S. U., Yun, B. S., Lee, S. J., Kim, J. H., and Yoo, I. D. (2002). Atroviridins A-C and neoatroviridins A-D, novel peptaibol antibiotics produced by Trichoderma atroviride F80317. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot.(Tokyo). 55[6], 557-564.
- 3047. Krysinska-Traczyk, E., Skorska, C., Cholewa, G., Sitkowska, J., Milanowski, J., and Dutkiewicz, J. (2002). Exposure to airborne microorganisms in furniture factories. Ann Agric Environ Med. 9[1], 85-90.
- 3266. Mackiewicz, B., Skorska, C., Krysinska-Traczyk, E., Larsson, L., Szponar, B., Milanowski, J., Czekajska-Chehab, E., Sitkowska, J., Cholewa, G., Szymanska, J., and Dutkiewicz, J. (2008). Respiratory disorders in two workers of customs depositories occupationally exposed to mouldy tobacco. Ann.Agric.Environ.Med. 15[2], 317-322.
- 3285. Federal Drug Administration (FDA). (2008). Biological products deviation reporting (BPDR). Non-blood product codes. 3-29-2009.
- 3318. UniProt Consortium. (2009). Taxonomy : fungi metazoa group. Site de UniProt . 4-6-2009.
- 3437. Reijula, K. and Tuomi, T. (2003). Mycotoxins of aspergilli: exposure and health effects. Front.Biosci. 8:s232-5., s232-s235.
- 3561. Ribes, J. A., Vanover-Sams, C. L., and Baker, D. J. (2000). Zygomycetes in human disease. Clin.Microbiol.Rev. 13[2], 236-301.
- 3670. Roussel, S., Reboux, G., Dalphin, J. C., Bardonnet, K., Millon, L., and Piarroux, R. (2004). Microbiological evolution of hay and relapse in patients with farmer's lung. Occup.Environ.Med. 61[1], e3.
- 3729. Flannigan, B., Samson, R. A., and Miller, J. D. (2002). Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. -504 p. CRC Press.
- 3839. Yman, L. (1992). Molds & Yeasts: Allergen related documents. ImmunoCAP InVitroSight , 1-3. Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden.
- 3840. Kwong, F. K. and Cook, B. (2002). The complete allergy book. Naperville, Ill, Sourcebooks.
- 3887. Stern, D. A., Morgan, W. J., Halonen, M., Wright, A. L., and Martinez, F. D. (2008). Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 372[9643], 1058-1064.
- 3899. Dutkiewicz, J., Skorska, C., Milanowski, J., Mackiewicz, B., Krysinska-Traczyk, E., Dutkiewicz, E., Matuszyk, A., Sitkowska, J., and Golec, M. (2001). Response of herb processing workers to work-related airborne allergens. Ann.Agric.Environ.Med. 8[2], 275-283.
- 3912. Simon-Nobbe, B., Denk, U., Poll, V., Rid, R., and Breitenbach, M. (2008). The spectrum of fungal allergy. Int.Arch.Allergy.Immunol. 145[1], 58-86.
- 3957. Krikstaponis, A., Lugauskas, A., Krysinska-Traczyk, E., Prazmo, Z., and Dutkiewicz, J. (2001). Enzymatic activities of Aspergillus fumigatus strains isolated from the air at waste landfills. Ann.Agric.Environ.Med. 8[2], 227-234.
- 3971. Robert, V., Stegehuis, G., and Stalpers, J. (2005). The MycoBank engine and related databases. International Mycological Association . International Mycological Association. 9-9-2009.
- 4093. Airaksinen, S., Heiskanen, M. L., Heinonen-Tanski, H., Laitinen, J., Laitinen, S., Linnainmaa, M., and Rautiala, S. (2005). Variety in dustiness and hygiene quality of peat bedding. Ann.Agric.Environ.Med. 12[1], 53-59.
- 4099. Amitani, R., Taylor, G., Elezis, E. N., Llewellyn-Jones, C., Mitchell, J., Kuze, F., Cole, P. J., and Wilson, R. (1995). Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect.Immun. 63[9], 3266-3271.
- 4100. Anaissie, E. J., Stratton, S. L., Dignani, M. C., Summerbell, R. C., Rex, J. H., Monson, T. P., Spencer, T., Kasai, M., Francesconi, A., and Walsh, T. J. (2002). Pathogenic Aspergillus species recovered from a hospital water system: a 3-year prospective study. Clin.Infect.Dis. 34[6], 780-789.
- 4102. Beffa, T., Staib, F., Lott, Fischer J., Lyon, P. F., Gumowski, P., Marfenina, O. E., Dunoyer-Geindre, S., Georgen, F., Roch-Susuki, R., Gallaz, L., and Latge, J. P. (1998). Mycological control and surveillance of biological waste and compost. Med Mycol. 36 Suppl 1:137-45., 137-145.
- 4103. Bergeron, V., Reboux, G., Poirot, J. L., and Laudinet, N. (2007). Decreasing airborne contamination levels in high-risk hospital areas using a novel mobile air-treatment unit. Infect.Control.Hosp.Epidemiol. 28[10], 1181-1186.
- 4104. Bertout, S., Badoc, C., Mallie, M., Giaimis, J., and Bastide, J. M. (2002). Spore diffusate isolated from some strains of Aspergillus fumigatus inhibits phagocytosis by murine alveolar macrophages. FEMS.Immunol.Med Microbiol. 33[2], 101-106.
- 4108. Comera, C., Andre, K., Laffitte, J., Collet, X., Galtier, P., and Maridonneau-Parini, I. (2007). Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes.Infect. 9[1], 47-54.
- 4110. Coyle, C. M., Kenaley, S. C., Rittenour, W. R., and Panaccione, D. G. (2007). Association of ergot alkaloids with conidiation in Aspergillus fumigatus. Mycologia. 99[6], 804-811.
- 4112. Cyprowski, M., Sowiak, M., Soroka, P. M., Buczyrnska, A., Kozajda, A., and Szadkowska-Stanczyk, I. (2008). [Assessment of occupational exposure to fungal aerosols in wastewater treatment plants]. Med Pr. 59[5], 365-371.
- 4113. Cyprowski, M., Buczynska, A., and Szadkowska-Stanczyk, I. (2006). [Exposure assessment to bioaerosols among sewer workers]. Med Pr. 57[6], 525-530.
- 4119. Ewig, S., Paar, W. D., Pakos, E., Schafer, H., Tasci, S., Marklein, G., and Luderitz, B. (1998). [Nosocomial ventilator-associated pneumonias caused by Aspergillus fumigatus in non-immunosuppressed, non-neutropenic patients]. Pneumologie. 52[2], 85-90.
- 4120. Frisvad, J. C., Rank, C., Nielsen, K. F., and Larsen, T. O. (2009). Metabolomics of Aspergillus fumigatus. Med Mycol. 47 Suppl 1:S53-71. Epub;%2008 Sep 1., S53-S71.
- 4125. Golec, M., Skorska, C., Mackiewicz, B., and Dutkiewicz, J. (2004). Immunologic reactivity to work-related airborne allergens in people occupationally exposed to dust from herbs. Ann.Agric.Environ.Med. 11[1], 121-127.
- 4128. Guarro, J., Sole, M., Castany, R., Cano, J., Teixido, A., Pujol, I., Gene, J., Castro, A., and Sarda, P. (2005). Use of random amplified microsatellites to type isolates from an outbreak of nosocomial aspergillosis in a general medical ward. Med Mycol. 43[4], 365-371.
- 4134. Hornef, M. W., Schopohl, J., Zietz, C., Hallfeldt, K. K., Roggenkamp, A., Gartner, R., and Heesemann, J. (2000). Thyrotoxicosis induced by thyroid involvement of disseminated Aspergillus fumigatus infection. J Clin.Microbiol. 38[2], 886-887.
- 4136. Kamei, K., Watanabe, A., Nishimura, K., and Miyaji, M. (2002). [Culture filtrate of Aspergillus fumigatus and its cytotoxic activity against leukocytes]. Nippon.Ishinkin.Gakkai.Zasshi. 43[3], 163-166.
- 4137. Kamei, K., Watanabe, A., Nishimura, K., and Miyaji, M. (2002). Cytotoxicity of Aspergillus fumigatus culture filtrate against macrophages. Nippon.Ishinkin.Gakkai.Zasshi. 43[1], 37-41.
- 4138. Karkowska-Kuleta, J., Rapala-Kozik, M., and Kozik, A. (2009). Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta.Biochim.Pol. 56[2], 211-224.
- 4139. Karvala, K., Nordman, H., Luukkonen, R., Nykyri, E., Lappalainen, S., Hannu, T., and Toskala, E. (2008). Occupational rhinitis in damp and moldy workplaces. Am.J Rhinol. 22[5], 457-462.
- 4140. Khoufache, K., Puel, O., Loiseau, N., Delaforge, M., Rivollet, D., Coste, A., Cordonnier, C., Escudier, E., Botterel, F., and Bretagne, S. (2007). Verruculogen associated with Aspergillus fumigatus hyphae and conidia modifies the electrophysiological properties of human nasal epithelial cells. BMC.Microbiol. 7:5., 5.
- 4143. Kupfahl, C., Michalka, A., Lass-Florl, C., Fischer, G., Haase, G., Ruppert, T., Geginat, G., and Hof, H. (2008). Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int.J Med Microbiol. 298[3-4], 319-327.
- 4144. Kurup, V. P., Kumar, A., Kenealy, W. R., and Greenberger, P. A. (1994). Aspergillus ribotoxins react with IgE and IgG antibodies of patients with allergic bronchopulmonary aspergillosis. J Lab.Clin.Med. 123[5], 749-756.
- 4145. Lasker, B. A. (2002). Evaluation of performance of four genotypic methods for studying the genetic epidemiology of Aspergillus fumigatus isolates. J Clin.Microbiol. 40[8], 2886-2892.
- 4146. Lewis, R. E., Wiederhold, N. P., Lionakis, M. S., Prince, R. A., and Kontoyiannis, D. P. (2005). Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J Clin.Microbiol. 43[12], 6120-6122.
- 4149. Madsen, A. M. (2006). Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann.Occup.Hyg. 50[8], 821-831.
- 4150. Mellado, E., az-Guerra, T. M., Cuenca-Estrella, M., Buendia, V., Aspa, J., Prieto, E., Villagrasa, J. R., and Rodriguez-Tudela, J. L. (2000). Characterization of a possible nosocomial aspergillosis outbreak. Clin.Microbiol.Infect. 6[10], 543-548.
- 4153. Munoz, P., Guinea, J., Pelaez, T., Duran, C., Blanco, J. L., and Bouza, E. (2004). Nosocomial invasive aspergillosis in a heart transplant patient acquired during a break in the HEPA air filtration system. Transpl.Infect.Dis. 6[1], 50-54.
- 4154. Neumann, H. D. and Balfanz, J. (1999). [Microbial exposure in collection of residential garbage--results of field studies]. Schriftenr.Ver.Wasser.Boden.Lufthyg. 104:533-45., 533-545.
- 4156. Nolard, N. (1996). [Invasive aspergillosis: nosocomial origin of epidemics. Review of the literature]. Bull.Acad.Natl.Med. 180[4], 849-856.
- 4159. Paugam, A., Renaud, B., Bousset, B., Salmon, D., and Dupouy-Camet, J. (1997). [Contribution of air mycological control for the prevention of invasive nosocomial aspergillosis]. Pathol.Biol.(Paris.). 45[5], 410-413.
- 4160. Pepeljnjak, S., Slobodnjak, Z., Segvic, M., Peraica, M., and Pavlovic, M. (2004). The ability of fungal isolates from human lung aspergilloma to produce mycotoxins. Hum.Exp.Toxicol. 23[1], 15-19.
- 4166. Rementeria, A., Lopez-Molina, N., Ludwig, A., Vivanco, A. B., Bikandi, J., Ponton, J., and Garaizar, J. (2005). Genes and molecules involved in Aspergillus fumigatus virulence. Rev.Iberoam.Micol. 22[1], 1-23.
- 4168. Ruiz-Hornillos, F. J., De Barrio, Fernandez M., Molina, P. T., Marcen, I. S., Fernandez, G. D., Sotes, M. R., and de Ocariz, M. L. (2007). Occupational asthma due to esparto hypersensitivity in a building worker. Allergy.Asthma.Proc. 28[5], 571-573.
- 4177. Skorska, C., Krysinska-Traczyk, E., Milanowski, J., Cholewa, G., Sitkowska, J., Gora, A., and Dutkiewicz, J. (2002). Response of furniture factory workers to work-related airborne allergens. Ann.Agric.Environ.Med. 9[1], 91-97.
- 4179. Spiewak, R., Skorska, C., Gora, A., Horoch, A., and Dutkiewicz, J. (2001). Young farmers with cellular reactivity to airborne microbes suffer more frequently from work-related skin symptoms and allergic dermatitis. Ann.Agric.Environ.Med. 8[2], 255-259.
- 4180. Spiewak, R., Gora, A., and Dutkiewicz, J. (2001). Work-related skin symptoms and type I allergy among eastern-Polish farmers growing hops and other crops. Ann.Agric.Environ.Med. 8[1], 51-56.
- 4186. Symoens, F., Viviani, M. A., and Nolard, N. (1993). Typing by immunoblot of Aspergillus fumigatus from nosocomial infections. Mycoses. 36[7-8], 229-237.
- 4189. Tomee, J. F. and Kauffman, H. F. (2000). Putative virulence factors of Aspergillus fumigatus. Clin.Exp.Allergy. 30[4], 476-484.
- 4193. Watanabe, A., Kamei, K., Sekine, T., Waku, M., Nishimura, K., Miyaji, M., and Kuriyama, T. (2003). Immunosuppressive substances in Aspergillus fumigatus culture filtrate. J Infect.Chemother. 9[2], 114-121.
- 4265. Vilavert, L., Nadal, M., Inza, I., Figueras, M. J., and Domingo, J. L. (2009). Baseline levels of bioaerosols and volatile organic compounds around a municipal waste incinerator prior to the construction of a mechanical-biological treatment plant. Waste.Manag. 29[9], 2454-2461.
- 4270. Anaissie, E. J., McGinnis, M. R., and Pfaller, M. A. (2003). Clinical mycology. Anaissie, E. J., McGinnis, M. R., and Pfaller, M. A. -608. Churchill Livingstone.
- 4285. Bartley, J. M. (2000). APIC state-of-the-Art report: the role of infection control during construction in health care facilities. Am.J Infect.Control. 28[2], 156-169.
- 4311. Tell, L. A. (2005). Aspergillosis in mammals and birds: impact on veterinary medicine. Med Mycol. 43 Suppl 1:S71-3., S71-S73.
- 4312. Karim, M., Alam, M., Shah, A. A., Ahmed, R., and Sheikh, H. (1997). Chronic invasive aspergillosis in apparently immunocompetent hosts. Clin.Infect.Dis. 24[4], 723-733.
- 4313. Saigal, G., Donovan Post, M. J., and Kozic, D. (2004). Thoracic intradural Aspergillus abscess formation following epidural steroid injection. AJNR.Am.J Neuroradiol. 25[4], 642-644.
- 4319. d'halewyn, M.-A. (1973). Infections aspergillaires de l'oreille externe et moyenne. Colloque de l'Association canadienne française pour l'avancement des sciences.








